Rhaponticin inhibits the proliferation, migration, and invasion of head and neck squamous cell carcinoma (HNSCC) cells through modulation of the IL6/STAT3 signaling pathway
- PMID: 40591033
- PMCID: PMC12214110
- DOI: 10.1007/s12672-025-03019-8
Rhaponticin inhibits the proliferation, migration, and invasion of head and neck squamous cell carcinoma (HNSCC) cells through modulation of the IL6/STAT3 signaling pathway
Abstract
Background: Rhaponticin, a bioactive compound derived from rhubarb, has been demonstrated anti-tumor effects in various types of cancer. However, its impact on HNSCC remains unexplored. This study aims to investigate the specific molecular mechanisms by which Rhaponticin inhibits the invasion and metastasis of HNSCC cells.
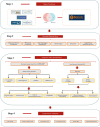
Method: The potential target genes that rhaponticin acts on in HNSCC were identified using online databases. The mechanisms by which rhaponticin influences the occurrence and progression of HNSCC were investigated through network pharmacology, molecular docking, bioinformatics analysis, and cellular experiments.
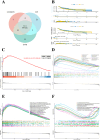
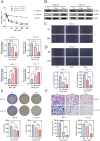
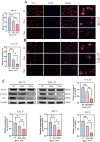
Result: Using network pharmacology, we identified 40 hub genes from the collected gene set. Subsequently, by analyzing The Cancer Genome Atlas (TCGA) data with four machine learning algorithms, we identified IL-6 as a potential target associated with the occurrence and progression of HNSCC. Based on the average expression level of IL-6, we classified the samples into high-expression and low-expression groups and conducted survival analysis. Our results indicated that IL-6 expression was significantly correlated with patient survival. Gene Set Enrichment Analysis (GSEA) revealed that Rhaponticin might influence HNSCC via the IL6/STAT3 signaling pathway. Using the CIBERSORT algorithm, we assessed the differences in infiltration levels of 22 immune cell types between the high and low IL-6 expression groups. Our findings suggest that multiple immune cells may be involved in the pathogenesis of HNSCC. Additionally, we analyzed single-cell RNA sequencing (scRNA-seq) data from the GEO database to compare IL6 expression levels in tumor and normal tissues and evaluated its prognostic impact using Receiver Operating Characteristic (ROC) curve analysis. Molecular docking studies demonstrated that Rhaponticin binds stably to IL6. In the experimental section, we used two HNSCC cell lines (CAL 27 and SCC-9) to investigate the effects of Rhaponticin. Our results showed that Rhaponticin effectively inhibited cell proliferation, invasion, and migration, and reduced the expression of proteins in the IL6/STAT3 signaling pathway.
Conclusion: Rhaponticin shows promise in treating HNSCC by inhibiting the IL6/STAT3 signaling pathway.
Keywords: Biomarkers; HNSCC; IL6/STAT3 signaling pathway; Inflammatory cytokines; Tumor immune infiltration.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors. Consent for publication: Not applicable. Consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Bioinformatics identification and validation of m6A/m1A/m5C/m7G/ac4 C-modified genes in oral squamous cell carcinoma.BMC Cancer. 2025 Jul 1;25(1):1055. doi: 10.1186/s12885-025-14216-7. BMC Cancer. 2025. PMID: 40597017 Free PMC article.
-
Identification of biomarkers for Laryngeal squamous cell carcinoma through Mendelian randomization and integrated bioinformatics analysis.Discov Oncol. 2025 Jul 18;16(1):1364. doi: 10.1007/s12672-025-03114-w. Discov Oncol. 2025. PMID: 40679719 Free PMC article.
-
Nuclear factor IA-mediated transcriptional regulation of crystallin αB inhibits hepatocellular carcinoma progression.Mol Clin Oncol. 2025 Jun 20;23(2):72. doi: 10.3892/mco.2025.2867. eCollection 2025 Aug. Mol Clin Oncol. 2025. PMID: 40599718 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F, Global Cancer S. 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. 10.3322/caac.21660. Epub 2021 Feb 4. PMID: 33538338. - PubMed
-
- Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez L, Wünsch-Filho V, Franceschi S, Hayes RB, Herrero R, Koifman S, La Vecchia C, Lazarus P, Levi F, Mates D, Matos E, Menezes A, Muscat J, Eluf-Neto J, Olshan AF, Rudnai P, Schwartz SM, Smith E, Sturgis EM, Szeszenia-Dabrowska N, Talamini R, Wei Q, Winn DM, Zaridze D, Zatonski W, Zhang ZF, Berthiller J, Boffetta P. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the international head and neck Cancer epidemiology consortium. J Natl Cancer Inst. 2007;99(10):777–89. 10.1093/jnci/djk179. Erratum in: J Natl Cancer Inst. 2008;100(3):225. Fernandez, Leticia [added]. PMID: 17505073. - PubMed
-
- Hunter KD, Parkinson EK, Harrison PR. Profiling early head and neck cancer. Nat Rev Cancer. 2005;5(2):127 – 35. 10.1038/nrc1549. PMID: 15685196. - PubMed
Grants and funding
- 82460954/National Natural Science Foundation of China
- 82460954/National Natural Science Foundation of China
- 82460954/National Natural Science Foundation of China
- 82460954/National Natural Science Foundation of China
- 82460954/National Natural Science Foundation of China
- 82460954/National Natural Science Foundation of China
- 82460954/National Natural Science Foundation of China
- 82460954/National Natural Science Foundation of China
- 20232BAB206153/Natural Science Foundation of Jiangxi Province
- 20232BAB206153/Natural Science Foundation of Jiangxi Province
- 20232BAB206153/Natural Science Foundation of Jiangxi Province
- 20232BAB206153/Natural Science Foundation of Jiangxi Province
- 20232BAB206153/Natural Science Foundation of Jiangxi Province
- 20232BAB206153/Natural Science Foundation of Jiangxi Province
- 20232BAB206153/Natural Science Foundation of Jiangxi Province
- 20232BAB206153/Natural Science Foundation of Jiangxi Province
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
