Safety and Immunogenicity of Monovalent Omicron KP.2-Adapted BNT162b2 COVID-19 Vaccine in Adults: Single-Arm Substudy from a Phase 2/3 Trial
- PMID: 40591130
- PMCID: PMC12339817
- DOI: 10.1007/s40121-025-01185-4
Safety and Immunogenicity of Monovalent Omicron KP.2-Adapted BNT162b2 COVID-19 Vaccine in Adults: Single-Arm Substudy from a Phase 2/3 Trial
Abstract
Introduction: COVID-19 continues to cause substantial health burden, particularly among vulnerable populations. Vaccines remain a vital tool in preventing severe disease outcomes. As the causative pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to evolve; therefore, updates may be needed to closely match COVID-19 vaccine composition to predominant circulating lineages to confer optimal protection.
Methods: In this cohort from a substudy of an ongoing phase 2/3 trial, 102 healthy adults (18‒55 and > 55 years of age, n = 51 each) were vaccinated with Omicron KP.2-adapted BNT162b2. Serum neutralizing titers against Omicron KP.2, JN.1, and KP.3 were assessed before and through 1 month after vaccination. Immunogenicity in KP.2-adapted BNT162b2 recipients was compared with participants who received JN.1-adapted BNT162b2 in an earlier cohort of this substudy. Local reactions and systemic events through 7 days and adverse events (AEs) through 1 month are reported.
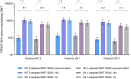
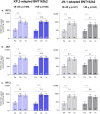
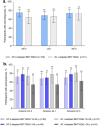
Results: One month after vaccination, KP.2-adapted BNT162b2-elicited neutralizing titers against Omicron KP.2, JN.1, and KP.3 were numerically higher than those induced by JN.1-adapted BNT162b2. Geometric mean fold rises from before to 1 month after vaccination were numerically higher in those who received KP.2-adapted BNT162b2 compared with those who received JN.1-adapted BNT162b2 (9.4 vs. 6.8 for KP.2; 7.8 vs. 5.7 for JN.1; 9.2 vs. 7.0 for KP.3). Percentages of participants with seroresponses were numerically higher against KP.2 after KP.2-adapted BNT162b2 than JN.1-adapted BNT162b2 (75% vs. 65%) and similar against JN.1 and KP.3 for both vaccines (69% vs. 67% for JN.1; 74% vs. 73% for KP.3). Local reactions and systemic events were all mild to moderate in severity, AEs were infrequent, and no serious AEs or AEs leading to withdrawal were reported.
Conclusions: Collectively, these immunogenicity, safety, and tolerability data support administration of KP.2-adapted BNT162b2 to protect against contemporaneous circulating lineages.
Gov identifier: NCT05997290.
Keywords: BNT162b2; Booster; COVID-19; Lineage; Omicron KP.2; SARS-CoV-2 vaccine; Sublineage; Variant adapted.
© 2025. Pfizer Inc.
Conflict of interest statement
Declarations. Conflicts of Interest: Federico Mensa, Özlem Türeci, and Uǧur Şahin are BioNTech employees and may hold stock or stock options. Özlem Türeci, Uǧur Şahin, Kena A. Swanson, and Kayvon Modjarrad report holding an interest in a patent relevant to this manuscript. Jing Zou, Xuping Xie, and Yanping Hu have received funding from Pfizer. Oyeniyi Diya is no longer an employee at Pfizer Ltd. All other authors (Juleen Gayed, Francine S. Lowry, Hua Ma, Vishva Bangad, Mark Cutler, Todd Belanger, David Cooper, Xia Xu, Robin Mogg, Annaliesa S. Anderson, Alejandra Gurtman and Nicholas Kitchin) are Pfizer employees and may hold stock or stock options. Ethical Approval: The study protocol was approved by the WCG Central institutional review board (approval number: 20233321; Princeton, NJ, USA), which was utilized by each study site. This substudy was conducted according to the protocol and consensus ethical principles derived from international guidelines (i.e., Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines), applicable good clinical practice guidelines of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, and other relevant laws and regulations. All participants were required to provide a signed statement of informed consent before study-specific procedures were performed.
Figures

References
-
- World Health Organization. COVID-19 advice for the public: getting vaccinated. WHO. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19.... Accessed 24 Jan 2025.
-
- Harris E. WHO declares end of COVID-19 global health emergency. JAMA. 2023;329:1817. - PubMed
-
- Markov PV, Ghafari M, Beer M, et al. The evolution of SARS-CoV-2. Nat Rev Microbiol. 2023;21:361–79. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

