Recent Advances in Synthetic Strategies and Biological Properties of Indazole Scaffolds: A Review
- PMID: 40591193
- PMCID: PMC12213994
- DOI: 10.1007/s41061-025-00509-9
Recent Advances in Synthetic Strategies and Biological Properties of Indazole Scaffolds: A Review
Abstract
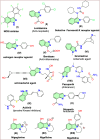
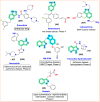
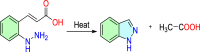
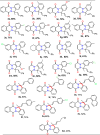
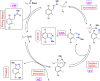
Research on heterocyclic compounds is an area of continuous focus, capturing the interest of both synthetic and natural product chemists. Indazoles are one of the rare heterocycles that are available in nature. Indazole and its derivatives are one of the most important classes of heterocycles in pharmacological molecules. The structurally different indazole motifs, with impressive bioactivity, have drawn increasing attention from medicinal chemists in recent years for the continuous development of novel drug moieties. Thus, knowledge of the biological activities and synthetic pathways of indazole scaffolds is essential to enhancing further developments in the number of indazole-based lead molecules. The goal of the present review is to highlight information on the biological properties of some existing indazole-based drugs and activities of novel bioactive indazole compounds in clinical trails, with specific attention to the most recent advances in various synthetic strategies towards indazole and its derivatives over the past 7 years (2017-2024). Moreover, we discuss the substrate tolerance and mechanistic insights for most of the summarized synthetic protocols.
Keywords: Biological activities; Clinical trails; Heterocycles; Indazole; Mechanistic insights; Synthetic routes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: The authors declare no competing interests.
Figures




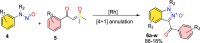




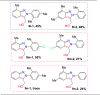
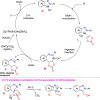



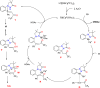
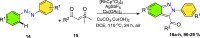


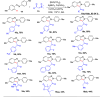
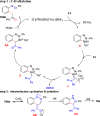

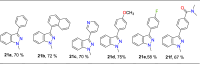
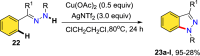
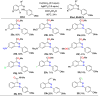
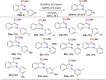

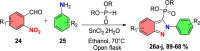
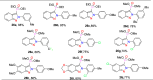

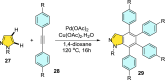
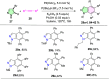
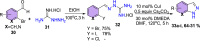

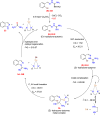

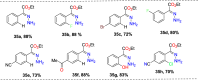
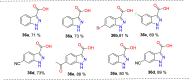





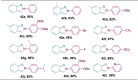
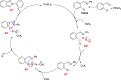

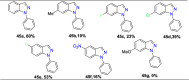
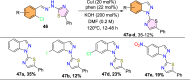
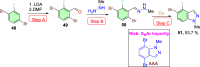

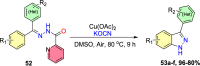
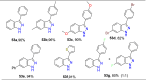
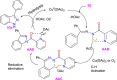
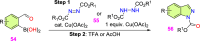
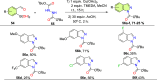
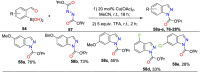
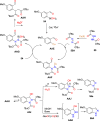
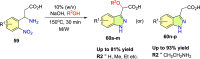
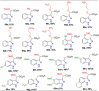

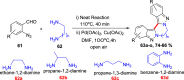

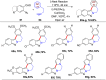
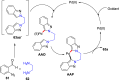

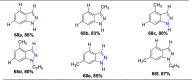

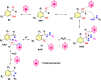
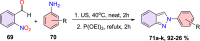
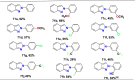
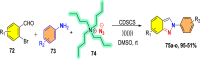
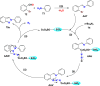
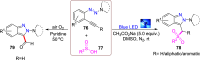
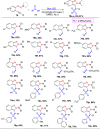
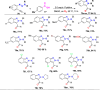



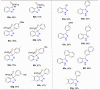
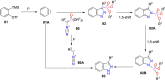

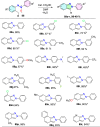
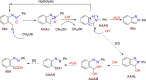


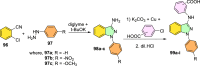
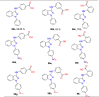

References
-
- Aaron DM, Musa ZN, Makhluf JH, Mark JK (2006) N,N-bond-forming heterocyclization: synthesis of 3-alkoxy-2H-indazoles. J Org Chem. 10.1021/jo0524831 - PubMed
-
- Thangadurai A, Minu M, Wakode S, Agarwal S, Narasimhan B (2012) Indazole: a medicinally important heterocyclic moiety. Med Chem Res. 10.1007/s00044-011-9631-3
-
- Catalan J, Elguero J (1987) Basicity and acidity of azoles. Adv Heterocyclic Chem. 10.1016/s0065-2725(08)60162-2
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
