Current insights into insect immune memory
- PMID: 40591395
- PMCID: PMC12212918
- DOI: 10.7554/eLife.105011
Current insights into insect immune memory
Abstract
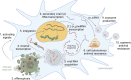
Traditionally, insects have been thought to be entirely dependent on their innate immune system, which has little capacity for the acquisition of experience from previous infections. However, much experimental evidence has challenged this view, showing that insects can develop long-term, pathogen-specific immune memory, which in some cases can be transmitted to offspring. Although significant progress has been made in this area, the underlying mechanism is still not fully understood, and a number of fundamental questions remain unanswered. In this review, we present an overview of documented cases of insect immune memory and summarize the experimental evidence in support of the prevailing hypotheses on the mechanism of antiviral and antibacterial immune memory in insects. We also highlight key questions that remain unanswered and discuss Drosophila melanogaster as a powerful model organism for investigating the mechanisms of innate immune memory formation. Finally, we evaluate the significance of this research and explore the potential for insect vaccination.
Keywords: Dscam; evolutionary biology; hemocytes; immune memory; immunology; inflammation; insect immunity; insect vaccination; trained immunity.
© 2025, Krejčová and Bajgar.
Conflict of interest statement
GK, AB No competing interests declared
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

