Impaired AMPK control of alveolar epithelial cell metabolism promotes pulmonary fibrosis
- PMID: 40591409
- PMCID: PMC12333953
- DOI: 10.1172/jci.insight.182578
Impaired AMPK control of alveolar epithelial cell metabolism promotes pulmonary fibrosis
Abstract
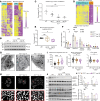
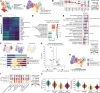
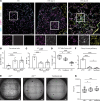
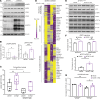
Alveolar epithelial type II (AT2) cell dysfunction is implicated in the pathogenesis of familial and sporadic idiopathic pulmonary fibrosis (IPF). We previously demonstrated that expression of an AT2 cell-exclusive disease-associated protein isoform (SP-CI73T) in murine and patient-specific induced pluripotent stem cell-derived (iPSC-derived) AT2 cells leads to a block in late macroautophagy and promotes time-dependent mitochondrial impairments; however, how a metabolically dysfunctional AT2 cell results in fibrosis remains elusive. Here, using murine and human iPSC-derived AT2 cell models expressing SP-CI73T, we characterize the molecular mechanisms governing alterations in AT2 cell metabolism that lead to increased glycolysis, decreased mitochondrial biogenesis, disrupted fatty acid oxidation, accumulation of impaired mitochondria, and diminished AT2 cell progenitor capacity manifesting as reduced AT2 cell self-renewal and accumulation of transitional epithelial cells. We identify deficient AMPK signaling as a critical component of AT2 cell dysfunction and demonstrate that targeting this druggable signaling hub can rescue the aberrant AT2 cell metabolic phenotype and mitigate lung fibrosis in vivo.
Keywords: Adult stem cells; Fibrosis; Metabolism; Mitochondria; Pulmonology.
Figures



Update of
-
Impaired AMPK Control of Alveolar Epithelial Cell Metabolism Promotes Pulmonary Fibrosis.bioRxiv [Preprint]. 2024 Mar 28:2024.03.26.586649. doi: 10.1101/2024.03.26.586649. bioRxiv. 2024. Update in: JCI Insight. 2025 Jul 1;10(15):e182578. doi: 10.1172/jci.insight.182578. PMID: 38585863 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- R01 HL119436/HL/NHLBI NIH HHS/United States
- F32 HL160011/HL/NHLBI NIH HHS/United States
- K08 HL150226/HL/NHLBI NIH HHS/United States
- U01 HL152976/HL/NHLBI NIH HHS/United States
- U01 HL134745/HL/NHLBI NIH HHS/United States
- R01 HL145408/HL/NHLBI NIH HHS/United States
- K99 HL171946/HL/NHLBI NIH HHS/United States
- R01 HL095993/HL/NHLBI NIH HHS/United States
- I01 BX001176/BX/BLRD VA/United States
- T32 HL007586/HL/NHLBI NIH HHS/United States
- K08 HL163494/HL/NHLBI NIH HHS/United States
- U01 HL134766/HL/NHLBI NIH HHS/United States
- 75N92020C00005/HL/NHLBI NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- P01 HL170952/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

