Targeting immune checkpoint LAIR1 with antibody blockade or 3-in-1 CAR T cells enhances antitumor response
- PMID: 40591413
- PMCID: PMC12352909
- DOI: 10.1172/JCI184043
Targeting immune checkpoint LAIR1 with antibody blockade or 3-in-1 CAR T cells enhances antitumor response
Abstract
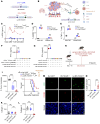
Tumor-associated macrophages (TAMs) are abundant in the tumor microenvironment (TME) and dampen the immune response, negatively affecting patient survival. Therefore, targeting TAMs could address the limitations of current cancer treatments. However, drug development in this area remains limited. The leukocyte-associated Ig-like receptor 1 (LAIR1), also called CD305, is prominently expressed on the surface of TAMs. We have uncovered what we believe to be a previously unrecognized immunosuppressive LAIR1/factor XIII A/collagen IV pathway across various cancer types. Inhibition of LAIR1, either through knockout (Lair1-/-), antibody blockade (anti-Lair1 antibody), or a chimeric antigen receptor (CAR) design (3-in-1 CAR by combining tumor targeting, T cell trafficking, and remodeling of the immunosuppressive TME in 1 CAR construct) provided an enhanced antitumor response. LAIR1 inhibition enhanced peripheral and intratumoral CD8 memory T cell populations, induced a phenotypic shift of M2-like macrophages toward M1 macrophages, and normalized tumor collagen IV and structural components in the TME, facilitating effective tumor-T cell interactions and tumor suppression. Enhanced antitumor responses were observed when Lair1-/- or anti-Lair1 antibody was used alone or in combination with CAR T cells or when the 3-in-1 CAR T cells were used solely in tumor models resistant to chemotherapy-radiation-programmed cell death protein 1 (PD-1) blockade. These findings position LAIR1 inhibition as a promising strategy for cancer immunotherapies.
Keywords: Cancer immunotherapy; Macrophages; Oncology; T cells; Therapeutics.
Conflict of interest statement
Figures





Comment in
- Targeting LAIR1-mediated immunosuppression adds a new weapon to our immunotherapy arsenal
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

