Cingulate retinoic acid signaling regulates neuropathic pain and comorbid anxiodepression via extracellular matrix homeostasis
- PMID: 40591414
- PMCID: PMC12404743
- DOI: 10.1172/JCI190539
Cingulate retinoic acid signaling regulates neuropathic pain and comorbid anxiodepression via extracellular matrix homeostasis
Abstract
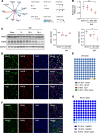
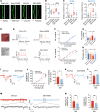
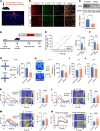
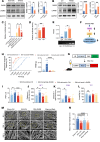
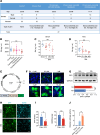
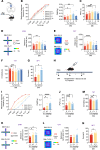
Neuropathic pain is often comorbid with affective disorders. Synaptic plasticity in anterior cingulate cortex (ACC) is assumed to be a crucial interface for pain perception and emotion. Laminin 1 (LAMB1), a key element of extracellular matrix (ECM) in ACC was recently revealed to convey extracellular alterations to intracellular synaptic plasticity and underlie neuropathic pain and aversive emotion. However, it remains elusive what triggers activity-dependent changes of LAMB1 and ECM remodeling after nerve injury. Here, we uncovered a key role of retinoic acid (RA)/RA receptor β (RARB) signaling in neuropathic pain and associated anxiodepression via regulation of ECM homeostasis. We showed that nerve injury reduced RA levels in the serum and ACC in mice and humans, which brought about downregulation of RA's corresponding receptor, RARB. Overexpressing RARB relieved pain hypersensitivity and comorbid anxiodepression, while silencing RARB exacerbated pain sensitivity and induced anxiodepression. Further mechanistic analysis revealed that RARB maintained ECM homeostasis via transcriptional regulation of LAMB1, reversing abnormal synaptic plasticity and eventually improving neuropathic pain and aversive emotion. Taken together with our previous study, we revealed an intracellular-extracellular-intracellular feed-forward regulatory network in modulating pain plasticity. Moreover, we identified cingulate RA/RARB signaling as a promising therapeutic target for treatment of neuropathic pain and associated anxiodepression.
Keywords: Cell biology; Extracellular matrix; Neuroscience; Pain; Synapses.
Figures





References
-
- Meng F, et al. Neural mechanisms of social empathy in the anterior cingulate cortex. Adv Neurol. 2023;2(1):281. doi: 10.36922/an.281. - DOI

