Identification of Functional Cellular Markers Related to Human Health, Frailty and Chronological Age
- PMID: 40591484
- PMCID: PMC12419852
- DOI: 10.1111/acel.70153
Identification of Functional Cellular Markers Related to Human Health, Frailty and Chronological Age
Abstract
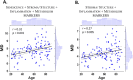
Aging leads to a decline in physiological reserves, an increase in age-related diseases, reduced functional ability and a shortened healthspan. While molecular markers of chronological aging exist, their link to general health and intrinsic capacity (IC), a composite measure of physical and mental capacities, remains unclear. This study integrates the WHO's Healthy Aging framework with geroscience to explore fibroblasts as indicators of health. We assessed primary skin fibroblasts from 133 individuals aged 20-96, evaluating their ability to maintain tissue structure, modulate immune responses and regulate metabolism (SIM functions). By combining functional and molecular analyses, we investigated the relationship between fibroblast performance, chronological age and IC. Our results demonstrate that fibroblast SIM functions are modified with stressors and age, correlating with IC rather than just chronological age. Notably, fibroblasts from pre-frail and frail individuals exhibited reduced mitochondrial respiration and lower extracellular periostin levels, with periostin being able to capture IC status, irrespective of age and sex, reflecting a cellular 'health memory'. The SIM paradigm provides a complementary framework to the established hallmarks of aging, advancing our understanding of how cellular aging impacts functional decline. These findings suggest that fibroblast-derived markers could serve as indicators of frailty and reduced IC, enabling early detection of individuals at risk for health deterioration and laying the foundation for early identification of functional decline.
Keywords: cellular aging; dermal fibroblast; extracellular matrix; healthy aging; intrinsic capacity; metabolism.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
- 1901175/INSPIRE Program
- MP0022856/INSPIRE Program
- ANR 23-IAHU-0011/IHU HealthAge of Toulouse (French National Research Agency (ANR) as part of the France 2030 program
- ANR-18-EURE-0003/EUR CARe
- PIA-ANR-11-INBS-005/Programme d'Investissements d'Avenir and the Agence Nationale pour la Recherche for the national infrastructure ECELLFrance
LinkOut - more resources
Full Text Sources
Medical

