Trastuzumab plus lapatinib or chemotherapy in patients with HER2-overexpressed advanced breast cancer: a randomized, phase II trial (GIM12-TYPHER)
- PMID: 40591514
- PMCID: PMC12265458
- DOI: 10.1093/oncolo/oyaf200
Trastuzumab plus lapatinib or chemotherapy in patients with HER2-overexpressed advanced breast cancer: a randomized, phase II trial (GIM12-TYPHER)
Abstract
Background: Trastuzumab combined with chemotherapy is a standard treatment for human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer in later lines. Lapatinib and trastuzumab have also demonstrated efficacy. This study assessed the efficacy, toxicity, and quality of life (QoL) of trastuzumab plus lapatinib (with endocrine therapy for hormone receptor-positive cases) versus trastuzumab with physician-selected chemotherapy in patients previously treated with at least 2 anti-HER2 regimens.
Methods: In this open-label, multicenter phase II trial, 59 patients were randomized 1:1 to receive either trastuzumab and lapatinib (arm A) or trastuzumab with chemotherapy (arm B). The primary endpoint was clinical benefit rate (CBR), defined as confirmed complete response, partial response, or stable disease for ≥24 weeks. Secondary endpoints included overall survival (OS), progression-free survival (PFS), overall response rate (ORR), QoL, and safety.
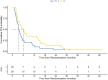
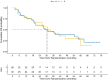
Results: With a median follow-up of 57.5 months, the CBR was 20.7% in arm A and 26.7% in arm B (P = .76). The ORR was 13.8% versus 20.0% (P = .73), and median PFS was 3.6 months in arm A versus 6.1 months in arm B (HR 0.63; P = .08). Median OS was 29.9 versus 31.1 months (HR 1.07; P = .82). Adverse events occurred in 86.2% (arm A) and 66.7% (arm B) of patients, with grade 3-4 events in 24.1% and 13.3%, respectively. QoL favored arm A (P = .03). Due to early study closure and limited sample size, all results should be considered exploratory and not powered to assess definitive treatment effects.
Conclusions: While efficacy differences were not significant, trastuzumab with lapatinib showed better QoL despite higher adverse event rates, suggesting it may be a viable chemotherapy-free option for pretreated HER2-positive advanced breast cancer.
Eudract trial registration number: 2013-005044-29.
Keywords: HER2; chemo-free treatment; lapatinib; metastatic breast cancer.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
C.D.A. reports consulting or advisory relationships with Novartis, GSK, Eli Lilly, and Pfizer. M.P. reports institutional funding from Gilead and travel reimbursement from Ipsen. M.M. reports consulting or advisory relationships with Amgen, AstraZeneca, Eli Lilly, Gilead, MSD, Novartis, Pfizer, and Seagen. M.D.L. reports a relationship with Roche, Novartis, Takeda, Lilly, Pierre Fabre, AstraZeneca, MSD, Seagen, Gilead, Daiichi Sankyo, Tomalab, Genetic, Pfizer, Menarini, Sophos, Istituto Gentili, Sanofi, Ipsen, GSK, and Exact Science that includes consulting or advisory and travel reimbursement. R.B. reports a relationship with Bayer, AstraZeneca, Sanofi, Novartis, Amgen, Roche, Pfizer, Janssen Cilag, and BMS that include consulting or advisory. R.C. reports a relationship with Novartis, Lilly, Daichii Sankyo, Veracyte, Pfizer, Roche, AstraZeneca, Seagen, MSD, Gilead, that include consulting or advisory and funding grants. S.C. is Fondazione AIOM president and reports a relationship with Lilly and Menarini Stemline that include consulting or advisory. L.D.M. reports a relationship with Lilly, Novartis, Roche, Menarini Stemline, Olema, GSK, Pfizer, Daiichi Sankyo, Exact Science, Gilead, Pierre Fabre, Eisai, AstraZeneca, Agendia, MSD, Seagen, and Ipsen that include consulting or advisory, funding grants, and travel reimbursement. F.P. reports a relationship with Amgen, AstraZeneca, Daichii Sankyo, Celgene, Eisai, Lilly, Exact Sciences, Italfarmaco, Menarini, Gilead, Ipsen, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, Takeda, and Viatris that include consulting or advisory, funding grants, and travel reimbursement. M.G. reports a relationship with AstraZeneca, Daichii Sankyo, Eisai, Gilead, Celgene, Exact Sciences, Lilly, MSD, Novartis, Pfizer, Roche, and Seagen that include consulting or advisory and travel reimbursement. G.A. reports a relationship with Roche, Pfizer, Lilly, MSD, AstraZeneca, and Novartis that include consulting or advisory and funding grants. The other authors did not declare any competing financial interests or personal relationships that could have influenced the work reported in this paper.
Figures
References
-
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; Published online 2021.
-
- Blaker H. Confidence curves and improved exact confidence intervals for discrete distributions. Can J Stat. 2000;28:783-798. https://doi.org/ 10.2307/3315916 - DOI
-
- Swain SM, Shastry M, Hamilton E.. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov. 2023;22:101-126. https://doi.org/ 10.1038/s41573-022-00579-0 - DOI - PMC - PubMed
-
- Di Maio M, Bighin C, Schettini F, et al. ; Gruppo Italiano Mammella (GIM) Study Group. Evolving treatments and outcomes in HER2-positive metastatic breast cancer: data from the GIM14/BIOMETA study. Breast. 2023;72:103583. https://doi.org/ 10.1016/j.breast.2023.103583 - DOI - PMC - PubMed
-
- Gennari A, André F, Barrios CH, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32:1475-1495. https://doi.org/ 10.1016/j.annonc.2021.09.019 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous