Cl-amidine confers organ protection and improves survival in hemorrhagic shock rats via the PAD4-CitH3-NETs axis
- PMID: 40591569
- PMCID: PMC12212502
- DOI: 10.1371/journal.pone.0327085
Cl-amidine confers organ protection and improves survival in hemorrhagic shock rats via the PAD4-CitH3-NETs axis
Abstract
Background: The occurrence of multi-organ dysfunction following hemorrhagic shock (HS) remains a critical clinical challenge. The excessive formation of neutrophil extracellular trap (NET) Has been identified as a pivotal pathogenic mechanism. This study preliminarily elucidated the protective mechanism of the PAD4 inhibitor Cl-amidine in a rat model of HS.
Methods: Male Sprague-Dawley rats were subjected to sublethal (40% blood loss, n = 8) or lethal (50% blood loss, n = 10) HS. Rats were divided into Sham group (catheter placement only), HS group (catheter placement followed by blood withdrawal), Vehicle group (0.9% saline), and Cl-amidine (10 mg/kg in 0.9% saline) groups.
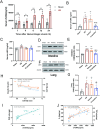
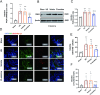
Results: Cl-amidine significantly improved the 72-h survival rate and delayed mortality in lethal HS. In Sublethal HS, the drug corrected metabolic disturbances, such as reduced lactate accumulation, while maintaining mean arterial pressure. Mechanistically, the effects of Cl-amidine included reducing circulating cell-free DNA (cf-DNA) and tissue citrullinated histone H3 (CitH3) levels, suppressing PAD4 expression, and improving histopathological outcomes (reduced edema and restored intestinal barrier integrity by upregulation of tight junction proteins Claudin-1/ZO-1). Moreover, Cl-amidine inhibited neutrophil infiltration through ICAM-1 downregulation and reduced the production of TNF-α and IL-6.
Conclusions: In conclusion, Cl-amidine protects against HS by targeting the PAD4-CitH3-NETs axis, breaking the vicious cycle of "NETs-inflammation", restoring barrier integrity, and alleviating multi-organ damage. The synergistic downregulation of ICAM-1 further enhances the therapeutic efficacy, highlighting Cl-amidine as a novel NETs-modulating strategy for HS. This study provides a theoretical and therapeutic foundation for the prevention and treatment of multi-organ injury following HS.
Copyright: © 2025 Yun et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exis.
Figures




Similar articles
-
Blocking peptidyl arginine deiminase 4 confers neuroprotective effect in the post-ischemic brain through both NETosis-dependent and -independent mechanisms.Acta Neuropathol Commun. 2025 Feb 18;13(1):33. doi: 10.1186/s40478-025-01951-y. Acta Neuropathol Commun. 2025. PMID: 39966968 Free PMC article.
-
Targeting PAD4: A Promising Strategy to Combat β-Cell Loss in Type 1 Diabetes.Int J Mol Sci. 2025 Jun 25;26(13):6113. doi: 10.3390/ijms26136113. Int J Mol Sci. 2025. PMID: 40649891 Free PMC article.
-
Redundant role of PAD2 and PAD4 in the development of cardiovascular lesions in a mouse model of Kawasaki disease vasculitis.Clin Exp Immunol. 2024 Nov 12;218(3):314-328. doi: 10.1093/cei/uxae080. Clin Exp Immunol. 2024. PMID: 39250707
-
Unraveling NETs in Sepsis: From Cellular Mechanisms to Clinical Relevance.Int J Mol Sci. 2025 Aug 1;26(15):7464. doi: 10.3390/ijms26157464. Int J Mol Sci. 2025. PMID: 40806591 Free PMC article. Review.
-
CitH3, a Druggable Biomarker for Human Diseases Associated with Acute NETosis and Chronic Immune Dysfunction.Pharmaceutics. 2025 Jun 23;17(7):809. doi: 10.3390/pharmaceutics17070809. Pharmaceutics. 2025. PMID: 40733019 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

