Altered auditory feature discrimination in a rat model of Fragile X Syndrome
- PMID: 40591708
- PMCID: PMC12237272
- DOI: 10.1371/journal.pbio.3003248
Altered auditory feature discrimination in a rat model of Fragile X Syndrome
Abstract
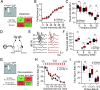
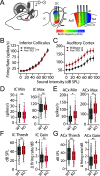
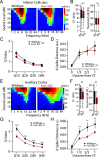
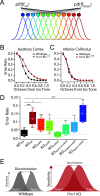
Atypical sensory processing, particularly in the auditory domain, is one of the most common and quality-of-life affecting symptoms seen in autism spectrum disorders (ASD). Fragile X Syndrome (FXS) is a leading inherited cause of ASD and a majority of FXS individuals present with auditory processing alterations. While auditory hypersensitivity is a common phenotype observed in FXS and Fmr1 knockout (KO) rodent models, it is important to consider other auditory coding impairments that could contribute to sound processing difficulties and disrupted language comprehension in FXS. We have shown previously that a Fmr1 KO rat model of FXS exhibits heightened sound sensitivity that coincided with abnormal perceptual integration of stimulus bandwidth, indicative of altered spectral processing. Frequency discrimination is a fundamental aspect of sound encoding that is important for a range of auditory processes, such as source segregation and speech comprehension, and disrupted frequency coding could thus contribute to a range of auditory issues in FXS and ASD. Here we explicitly characterized spectral processing deficits in male Fmr1 KO rats using an operant conditioning tone discrimination assay and in vivo electrophysiological recordings from the auditory cortex and inferior colliculus. We found that Fmr1 KO rats exhibited poorer frequency resolution, which corresponded with neuronal hyperactivity and broader frequency tuning in auditory cortical but not collicular neurons. Using an experimentally informed population model, we show that these cortical physiological differences can recapitulate the observed behavior discrimination deficits, with decoder performance being tightly linked to differences in cortical tuning width and signal-to-noise ratios. Together, these findings indicate that cortical hyperexcitability in Fmr1 KO rats may act to preserve signal-to-noise ratios and signal detection threshold at the expense of sound sensitivity and fine feature discrimination, highlighting a potential mechanistic locus for a range of auditory behavioral phenotypes in FXS.
Copyright: © 2025 Gauthier et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Update of
-
Altered auditory feature discrimination in a rat model of Fragile X Syndrome.bioRxiv [Preprint]. 2025 Feb 19:2025.02.18.638956. doi: 10.1101/2025.02.18.638956. bioRxiv. 2025. Update in: PLoS Biol. 2025 Jul 1;23(7):e3003248. doi: 10.1371/journal.pbio.3003248. PMID: 40027738 Free PMC article. Updated. Preprint.
Similar articles
-
Altered auditory feature discrimination in a rat model of Fragile X Syndrome.bioRxiv [Preprint]. 2025 Feb 19:2025.02.18.638956. doi: 10.1101/2025.02.18.638956. bioRxiv. 2025. Update in: PLoS Biol. 2025 Jul 1;23(7):e3003248. doi: 10.1371/journal.pbio.3003248. PMID: 40027738 Free PMC article. Updated. Preprint.
-
Phenotypic analysis of multielectrode array EEG biomarkers in developing and adult male Fmr1 KO mice.Neurobiol Dis. 2024 Jun 1;195:106496. doi: 10.1016/j.nbd.2024.106496. Epub 2024 Apr 4. Neurobiol Dis. 2024. PMID: 38582333
-
Septotemporal Variation of Information Processing in the Hippocampus of Fmr1 KO Rat.Dev Neurosci. 2024;46(6):353-364. doi: 10.1159/000537879. Epub 2024 Feb 16. Dev Neurosci. 2024. PMID: 38368859 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systematic Review: Fragile X Syndrome Across the Lifespan with a Focus on Genetics, Neurodevelopmental, Behavioral and Psychiatric Associations.Genes (Basel). 2025 Jan 25;16(2):149. doi: 10.3390/genes16020149. Genes (Basel). 2025. PMID: 40004478 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

