Pseudonatural Products for Chemical Biology and Drug Discovery
- PMID: 40591938
- PMCID: PMC12305498
- DOI: 10.1021/acs.jmedchem.5c00643
Pseudonatural Products for Chemical Biology and Drug Discovery
Erratum in
-
Correction to "Pseudonatural Products for Chemical Biology and Drug Discovery".J Med Chem. 2025 Oct 21. doi: 10.1021/acs.jmedchem.5c02912. Online ahead of print. J Med Chem. 2025. PMID: 41117564 No abstract available.
Abstract
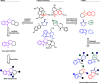
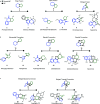
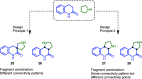
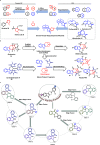
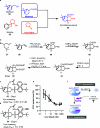
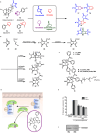
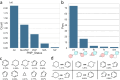
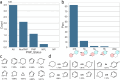
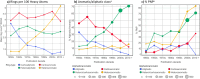
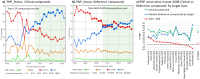
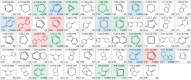
Natural product (NP) structures have provided invaluable inspiration for the discovery of bioactive compound discovery. In the pseudonatural product (PNP) concept unprecedented combinations of NP fragments combine the biological relevance of NPs with exploration of wider chemical space by fragment-based design. We describe the principles underlying the PNP design and discovery of selected PNPs with unexpected or novel bioactivity. Cheminformatic analyses of ChEMBL 32, the Enamine screening library, phase 1-3 clinical compounds, and approved drugs reveal that ca. 1/3 of historically developed biologically active compounds and of currently commercially available screening compounds are PNPs, and that PNPs are increasing over time. PNPs are 54% more likely to be found in clinical compounds versus nonclinical compounds, and 67% of recent clinical compounds are PNPs. 63% of the core scaffolds in recent clinical compounds are made up of just 176 NP fragments, which suggests that PNPs open up a multitude of unexploited opportunities for drug discovery.
Figures








References
-
- Makurvet F. D.. Biologics vs. small molecules: Drug costs and patient access. Med. Drug Discovery. 2021;9:100075. doi: 10.1016/j.medidd.2020.100075. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

