Trajectory of plasma lipidome associated with the risk of late-onset Alzheimer's disease: a longitudinal cohort study
- PMID: 40592256
- PMCID: PMC12269576
- DOI: 10.1016/j.ebiom.2025.105826
Trajectory of plasma lipidome associated with the risk of late-onset Alzheimer's disease: a longitudinal cohort study
Abstract
Background: Comprehensive lipidomic studies have demonstrated strong cross-sectional associations between the blood lipidome and late-onset Alzheimer's disease (AD) dementia and its risk factors, yet the longitudinal relationship between lipidome changes and AD progression remains unclear.
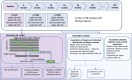
Methods: We employed longitudinal lipidomic profiling on 4730 plasma samples from 1517 participants of the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort to investigate the temporal evolution of lipidomes among diagnostic groups. At baseline (n = 1393), participants were classified as stable diagnosis status including stable AD (n = 243), stable cognitive normal (CN; n = 337), and stable mild cognitive impairment (MCI; n = 413), or converters (AD converters: n = 329; MCI converters: n = 71). We developed a dementia risk classification model to stratify the non-converting MCI group into dementia-like and non-dementia-like MCI based on their baseline lipidomic profiles, aiming to identify early metabolic signatures predictive of dementia progression.
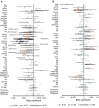
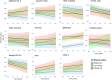
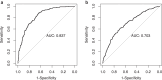
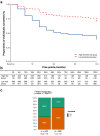
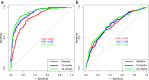
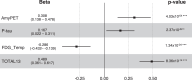
Findings: Longitudinal analysis identified significant associations between the change in ether lipid species (including alkylphosphatidylcholine, alkenylphosphatidylcholine, lysoalkylphosphatidylcholine, and lysoalkenylphosphatidylcholine) and AD dementia conversion. Specifically, AD dementia converters show a 3-4.8% reduction in these ether lipid species compared to the non-converting CN and MCI groups, suggesting metabolic dysregulation as a key feature of AD progression. Further, The Dementia Risk Model effectively distinguished MCI from AD dementia converters (AUC = 0.70; 95% CI: 0.66-0.74). Within the MCI group, the model identified a high-risk subgroup with a twofold higher likelihood of conversion to AD dementia compared to the low-risk group. External validation in the ASPREE cohort confirmed its predictive utility, with the Dementia Risk Score discriminating incident dementia from cognitively normal individuals (C-index = 0.75, 95% CI: 0.73-0.78), improving prediction by 2% over the combination of traditional risk factors and APOE genetic risk factor. Additionally, the Dementia Risk Score was significantly associated with reduced temporal lobar fludeoxyglucose uptake (β = -0.286, p = 1.34 × 10-4), higher amyloid PET levels (β = 0.308, p = 4.03 × 10-4), and elevated p-tau levels (β = 0.167, p = 2.37 × 10-2), reinforcing its pathophysiological relevance in tracking neurodegeneration, amyloid burden, and tau pathology.
Interpretation: These findings highlight lipidomic profiling as a potential blood-based biomarker for identifying individuals at high risk of AD progression, offering a scalable, non-invasive approach for early detection, risk stratification, and targeted interventions in AD.
Funding: The National Health and Medical Research Council of Australia (#1101320 and #1157607); NHMRC Investigator grant (#GNT1197190); Victorian Government's Operational Infrastructure Support Program; National Heart Foundation of Australia, Future Leader Fellowship (#102604), and National Health and Medical Research Council Investigator Grant (#2026325); Investigator grant (#2009965) from the National Health and Medical Research Council of Australia; a National Health and Medical Research Council of Australia Senior Research Fellowship (#1042095); National Institutes of Health grants: P30AG010133, P30AG072976, R01AG019771, R01AG057739, U19AG024904, R01LM013463, R01AG068193, T32AG071444, U01AG068057, U01AG072177, U19AG074879, R01AG069901, R01AG046171, RF1AG051550, RF1AG057452; National Institutes of Health/National Institute on Aging grants RF1AG058942, RF1AG059093, U01AG061359, U19AG063744, and R01AG081322, NIH/NLM R01LM012535; FNIH: DAOU16AMPA.
Keywords: AD biomarkers; Alzheimer's disease; Cognitively normal; Lipidomics; Mild cognitive impairment.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Dr. Kaddurah-Daouk is an inventor on a series of patents on use of metabolomics for the diagnosis and treatment of CNS diseases and holds equity in Metabolon Inc., Chymia LLC and PsyProtix. Prof. Meikle leads the provisional patent “METHODS OF ASSESSING ALZHEIMER’S DISEASE” on the development of dementia risk scores that has been filed with the Serial No. 63/463,808. Gabi Kastenmüller declares equity in Chymia LLC and EMBL-EBI, is the inventor on patents, Philippine Genome Center/Davao Medical School, DAAD/DWIH, and BMBF. Andrew Saykin has received gifts/services from Avid Radiopharmaceuticals, has editorial involvement with Springer-Nature Publishing, and has served on scientific advisory boards for Eisai, Novo Nordisk, and Siemens. Paul Lacaze is supported by a National Heart Foundation of Australia Future Leader Fellowship. Matthias Arnold reports support from NIH/NIA, is a co-inventor on patents, and has equity in Chymia LLC, PsyProtiz, and Atai Life Sciences. Other authors have declared that no conflict of interest exists.
Figures

References
-
- Niccoli T., Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22(17):R741–R752. - PubMed
-
- van der Lee S.J., Wolters F.J., Ikram M.K., et al. The effect of APOE and other common genetic variants on the onset of Alzheimer's disease and dementia: a community-based cohort study. Lancet Neurol. 2018;17(5):434–444. - PubMed
-
- Weidner W.S., Barbarino P. P4-443: the state of the art of dementia research: new frontiers. Alzheimers Dement. 2019;15(7S_Part_28):P1473.
-
- Bowler J.V. The concept of vascular cognitive impairment. J Neurol Sci. 2002;203:11–15. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

