Electronic medicine management systems in developing countries: A landscape review
- PMID: 40592719
- PMCID: PMC12381608
- DOI: 10.1002/bcp.70156
Electronic medicine management systems in developing countries: A landscape review
Abstract
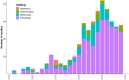
Medicines are a major global health expense. However, suboptimal use increases costs and causes patient harm. One way to reduce costs and increase safe, efficient medicines use is with electronic medicines management systems (EMMS). They allow easy capture of routine health data which can facilitate research, service planning and reimbursement processes. There are various barriers to healthcare digitization in developing countries (DCs), although some have overcome these. We sought to understand the landscape of EMMS use in DCs. We systematically searched six bibliographic databases from inception to 23 October 2024 for studies reporting the implementation and/or use of EMMS in countries with lower than 'very high' Human Development Index (HDI). We qualitatively and quantitatively summarized data on geographic location, healthcare setting and system functionality. We created an interactive map illustrating spatial and temporal trends in EMMS use. A total of 314 records described the use of EMMS in 45 DCs, 206 of which described coexistence/integration of other health data (e.g., electronic health records [EHR]). Predominantly, EMMS were for prescribing (n = 264) or dispensing (n = 66), implemented in secondary care settings and operated locally rather than regionally or nationally. Common EMMS use-cases included adherence monitoring in human immunodeficiency virus (HIV) and tuberculosis treatment. Our findings highlight both widespread EMMS adoption-commonly in the context of a broader EHR-and persistent gaps in implementation. These insights could be used by policymakers and healthcare leaders to guide strategy and funding decisions. Existing systems could be leveraged for service planning, healthcare delivery and optimizing medicine use. Where EMMS are not yet in use, our findings provide a roadmap for stakeholders to identify and emulate successful implementations in similar healthcare settings. Expanding the interoperability and scale of EMMS could further enable transformative digital technologies, increasing efficiencies and coverage, and ultimately improving patient outcomes.
Keywords: developing countries; digital; electronic; low‐ and middle‐income countries; medicine management; review.
© 2025 The Author(s). British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest relating to this work.
Figures



References
-
- World Health Organization . Global spending on health. World Health Organization; 2022.
-
- World Health Organization . Medication without harm. World Health Organization; 2017.
-
- García‐Goñi M Rationalizing pharmaceutical spending 2022 IMF Working Paper 2022 190 doi: 10.5089/9798400219849.001 - DOI
-
- Franklin BD, O'Grady K, Donyai P, Jacklin A, Barber N. The impact of a closed‐loop electronic prescribing and administration system on prescribing errors, administration errors and staff time: a before‐and‐after study. Qual Saf Health Care. 2007;16(4):279‐284. doi: 10.1136/qshc.2006.019497 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

