Mitigation and management of adverse events associated with amivantamab therapy
- PMID: 40592737
- PMCID: PMC12282346
- DOI: 10.1093/oncolo/oyaf194
Mitigation and management of adverse events associated with amivantamab therapy
Abstract
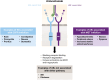
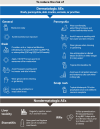
Amivantamab is a fully human bispecific epidermal growth factor receptor (EGFR)-directed and mesenchymal epithelial transition (MET) receptor-directed antibody. Intravenous amivantamab is approved and recommended by treatment guidelines as a first-line treatment (1L) in combination with lazertinib, as a second-line treatment (2L) in combination with chemotherapy in adults with advanced or metastatic non-small cell lung cancer (NSCLC) with EGFR exon 19 deletions or exon 21 L858R substitution mutations, and as 2L monotherapy or 1L in combination with chemotherapy in adults with advanced or metastatic NSCLC with exon 20 insertion-mutations. Compared with previous therapies, novel treatments such as amivantamab may be associated with distinct and unique adverse reactions that potentially require optimized prevention and management techniques. Commonly reported adverse reactions associated with amivantamab treatment regimens include cutaneous reactions associated with EGFR inhibition, such as rash, paronychia, and pruritus; those associated with MET inhibition, such as peripheral edema and hypoalbuminemia; and general effects, such as infusion-related reactions. Recommendations are summarized from published guidelines and the authors' clinical experience for the prevention and management of adverse reactions associated with amivantamab. An understanding of the expected adverse events with amivantamab regimens, along with the range of prophylactic and management options available, may facilitate maintenance of ongoing treatment in patients deriving clinical benefit and improve patient quality of life on therapy.
Keywords: adverse drug reaction; epidermal growth factor receptor; mesenchymal epithelial transition; non-small cell lung cancer.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
NF reports consulting fees or honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Jazz Pharmaceuticals, Johnson & Johnson, Novocure, Pfizer, Sanofi and Takeda; travel from AstraZeneca, Pfizer, Johnson & Johnson, and Takeda; and research funding from Genentech, Daiichi Sankyo, AstraZeneca and Johnson & Johnson. NRL reports no conflicts of interest. JR reports consulting fees or honoraria from: Amgen, AstraZeneca, BioAtla, Bristol Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, EMD Serono, Genentech, G1 Therapeutics, Guardant Health, Johnson and Johnson, Jazz Pharmaceuticals, Merus, Novocure, Pfizer, Sanofi-Genzyme, Summit Therapeutics, and Takeda. Travel from AstraZeneca, Bristol Myers Squibb, Johnson and Johnson, and Merus. Contracted for research (institutional) with: Altor Bioscience, AstraZeneca, Bicycle Therapeutics, BioAtla, Blueprint Medicines, Enliven Therapeutics, EpimAb, Black Diamond, Duality, LOXO Oncology, ORIC Pharmaceuticals, AbbVie, RedCloud Bio, Summit, Synthekine, Immunity Bio, and Regeneron. JAM reports consulting fees or honoraria from Curio Science, Daiichi Sankyo, Gather-Ed, Johnson & Johnson, and Merus; travel from IDEOlogy Health, Merus, MJH Life Sciences, PrecisCa, and Targeted Healthcare Communications. JKS reports consulting or advising fees from AstraZeneca, Abbvie, EMD Serono, Genentech, Janssen Johnson and Johnson, Jazz, Loxo, Lilly, Mirati/Bristol Myers Squibb, Pfizer, Regeneron, Revolution Medicines, Sanofi Genzyme, and Takeda; and research support (institutional) from Janssen, Johnson and Johnson, Loxo, Lilly, Mirati/Bristol Myers Squibb, Regeneron, and ORIC. OA reports consulting or advising fees from AstraZeneca, Bristol Myers Squibb, Lilly, Merck, and Roche; honoraria from AstraZeneca, Boehringer Ingelheim, Pfizer, and Roche; research funding (institutional) from AstraZeneca, Bristol Myers Squibb, Merck, and Roche; and travel, accommodations, and/or expenses from Lilly. CB reports consulting fees from Amgen, AstraZeneca, Janssen, Merck, Pfizer, and Roche; payment or honoraria from Amgen, AstraZeneca, Janssen, Merck, Pfizer, Roche, and Takeda; payment for expert testimony from AstraZeneca; support for meetings and/or travel from Johnson and Johnson, Pfizer, Roche, and Servier; participation on a data safety monitoring or advisory board for AstraZeneca, Johnson and Johnson, and Pfizer; and leadership or fiduciary role with Brazilian Society of Clinical Oncology and IASLC. RG reports consulting fees from AstraZeneca and Gilead; honoraria from Cor2Ed, SignifyMD, MJH Life Sciences, and Integrity CE; advisory board fees from AstraZeneca; and cofounder of Oncology Brothers Podcast. DZ reports honoraria from Johnson and Johnson. SM reports being a consultant for AstraZeneca, Bristol Myers Squibb, and Pfizer. ED and PC report being employees of Johnson & Johnson. BM reports research funding from Bristol Myers Squibb; research support from Paxman; and serving on an advisory board for Daiichi Sankyo. NRL reports being a consultant and receiving honoraria from Bayer, Fortress Biotech, Sanofi, Seagen, Silverback Therapeutics, Synox Therapeutics, and Janssen and Astellas pharma outside the scope of the submitted work.
None declared.
Figures




References
-
- Somme LB, Chouaid C, Moinard-Butot F, et al. Antibody-drug conjugates as novel therapeutic agents for non-small cell lung carcinoma with or without alterations in oncogenic drivers. BioDrugs. 2024;38:487-497. https://doi.org/ 10.1007/s40259-024-00660-7 - DOI - PMC - PubMed
-
- Efil SC, Bilgin B, Ceylan F, et al. A current comprehensive role of immune-checkpoint inhibitors in resectable non-small cell lung cancer: a narrative review. J Oncol Pharm Pract. 2024;30:1214-1239. https://doi.org/ 10.1177/10781552241260864 - DOI - PubMed
-
- Xu J, Tian L, Qi W, Lv Q, Wang T.. Advancements in NSCLC: from pathophysiological insights to targeted treatments. Am J Clin Oncol. 2024;47:291-303. https://doi.org/ 10.1097/COC.0000000000001088 - DOI - PMC - PubMed
-
- Pao W, Girard N.. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175-180. https://doi.org/ 10.1016/S1470-2045(10)70087-5 - DOI - PubMed
-
- Janne PA, Johnson BE.. Effect of epidermal growth factor receptor tyrosine kinase domain mutations on the outcome of patients with non-small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2006;12:4416s-4420s. https://doi.org/ 10.1158/1078-0432.CCR-06-0555 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

