A biomimetic multi-component subunit vaccine via ratiometric loading of hierarchical hydrogels
- PMID: 40592824
- PMCID: PMC12218367
- DOI: 10.1038/s41467-025-60416-x
A biomimetic multi-component subunit vaccine via ratiometric loading of hierarchical hydrogels
Abstract
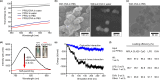
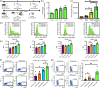
The development of subunit vaccines that mimic the molecular complexity of attenuated vaccines has been limited by the difficulty of intracellular co-delivery of multiple chemically diverse payloads at controllable concentrations. We report on hierarchical hydrogel depots employing simple poly(propylene sulfone) homopolymers to enable ratiometric loading of a protein antigen and four physicochemically distinct adjuvants in a hierarchical manner. The optimized vaccine consisted of immunostimulants either adsorbed to or encapsulated within nanogels, which were capable of noncovalent anchoring to subcutaneous tissues. In female BALB/c and C57BL/6 mice, these 5-component nanogel vaccines demonstrated enhanced humoral and cell-mediated immune responses compared to formulations with standard single adjuvant and antigen pairing. The use of a single simple homopolymer capable of rapid and stable loading and intracellular delivery of diverse molecular cargoes holds promise for facile development and optimization of scalable subunit vaccines and complex therapeutic formulations for a wide range of biomedical applications.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Update of
-
A Biomimetic Multi-Component Subunit Vaccine via Ratiometric Loading of Hierarchical Hydrogels.Res Sq [Preprint]. 2024 Apr 25:rs.3.rs-4177821. doi: 10.21203/rs.3.rs-4177821/v1. Res Sq. 2024. Update in: Nat Commun. 2025 Jul 1;16(1):5443. doi: 10.1038/s41467-025-60416-x. PMID: 38746232 Free PMC article. Updated. Preprint.
Similar articles
-
A Biomimetic Multi-Component Subunit Vaccine via Ratiometric Loading of Hierarchical Hydrogels.Res Sq [Preprint]. 2024 Apr 25:rs.3.rs-4177821. doi: 10.21203/rs.3.rs-4177821/v1. Res Sq. 2024. Update in: Nat Commun. 2025 Jul 1;16(1):5443. doi: 10.1038/s41467-025-60416-x. PMID: 38746232 Free PMC article. Updated. Preprint.
-
A human cytomegalovirus prefusion-like glycoprotein B subunit vaccine elicits humoral immunity similar to that of postfusion gB in mice.J Virol. 2025 Jun 17;99(6):e0217824. doi: 10.1128/jvi.02178-24. Epub 2025 May 8. J Virol. 2025. PMID: 40338082 Free PMC article.
-
Flagellin Enhances the Immunogenicity of Pasteurella multocida Lipoprotein E Subunit Vaccine.Avian Dis. 2024 Sep;68(3):183-191. doi: 10.1637/aviandiseases-D-24-00032. Avian Dis. 2024. PMID: 39400212
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
References
-
- Reed, S. G., Orr, M. T. & Fox, C. B. Key roles of adjuvants in modern vaccines. Nat. Med.19, 1597–1608 (2013). - PubMed
-
- Walvekar, P., Kumar, P. & Choonara, Y. E. Long-acting vaccine delivery systems. Adv. Drug Deliv. Rev.198, 114897 (2023). - PubMed
-
- Ou, B. S., Saouaf, O. M., Baillet, J. & Appel, E. A. Sustained delivery approaches to improving adaptive immune responses. Adv. Drug Deliv. Rev.187, 114401 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

