Integrated dopamine sensing and 40 Hz hippocampal stimulation improves cognitive performance in Alzheimer's mouse models
- PMID: 40592843
- PMCID: PMC12217251
- DOI: 10.1038/s41467-025-60903-1
Integrated dopamine sensing and 40 Hz hippocampal stimulation improves cognitive performance in Alzheimer's mouse models
Abstract
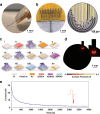
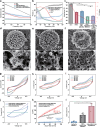
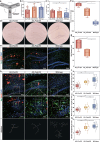
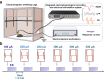
Hippocampal degeneration and reduced dopamine levels in Alzheimer's disease are associated with severe memory and cognitive impairments. However, the lack of multifunctional in situ neural chips has posed challenges for integrated investigations of Alzheimer's disease pathophysiology, dopamine dynamics, and neural activity. Therefore, we developed NeuroRevive-FlexChip, a flexible neural interface capable of precise electrical modulation and simultaneous in situ monitoring of dopamine levels and neural activity. In this study, the NeuroRevive-FlexChip demonstrates improved electrochemical detection sensitivity and modulation efficiency. Preliminary observations in APP/PS1 mice indicate that implantation of the chip in the hippocampal CA1 region, combined with 40 Hz stimulation, may contribute to the restoration of dopamine release, a reduction in neuronal hyper-synchronization, and a shift toward more stable firing patterns. These effects appear to be modulated by dopamine-related mechanisms. Furthemore, 40 Hz stimulation was observed to correlate with reduction in Aβ42 deposition and modest improvements in spatial cognition performance, as assessed by the Y-maze test. These findings highlight the potential of NeuroRevive-FlexChip as a research tool for investigating the mechanisms of 40 Hz stimulation in Alzheimer's disease models. Further studies could explore its utility in clarifying the relationship between dopamine dysfunction, neural activity, and amyloid pathology. While these early results are promising, additional preclinical and translational research will be necessary to assess the therapeutic potential of this approach for neurodegenerative diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.L., X.C., Y.S., F.M., L.J., W.X. are involved in the patent ‘Flexible Neural Electrode with Multilayer Bidirectional Signal Transmission Structure and Its Fabrication Method’. The other authors declare no competing interests. Ethics: All animal care and procedures adhered to the guidelines set by the Institutional Animal Care and Use Committee at the Aerospace Information Research Institute, Chinese Academy of Science (AIRCAS), and were approved by the Beijing Laboratory Animal Care Association and the AIRCAS Animal Care and Use Committee under approval number AIRCAS-202103-1.
Figures



Similar articles
-
Intermittent ELF-MF exposure effectively ameliorates pathologic features associated with adult AD mice.Brain Res. 2025 Oct 1;1864:149796. doi: 10.1016/j.brainres.2025.149796. Epub 2025 Jun 20. Brain Res. 2025. PMID: 40544930
-
GALM Alleviates Aβ Pathology and Cognitive Deficit Through Increasing ADAM10 Maturation in a Mouse Model of Alzheimer's Disease.Neurosci Bull. 2025 Aug;41(8):1377-1389. doi: 10.1007/s12264-025-01386-4. Epub 2025 Mar 24. Neurosci Bull. 2025. PMID: 40128515
-
Vascular dysfunction occurs prior to the onset of amyloid pathology and Aβ plaque deposits colocalize with endothelial cells in the hippocampus of female APPswe/PSEN1dE9 mice.Geroscience. 2024 Dec;46(6):5517-5536. doi: 10.1007/s11357-024-01213-0. Epub 2024 Jun 11. Geroscience. 2024. PMID: 38862757 Free PMC article.
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
Systematic review of the relationship between amyloid-β levels and measures of transgenic mouse cognitive deficit in Alzheimer's disease.J Alzheimers Dis. 2015;44(3):787-95. doi: 10.3233/JAD-142208. J Alzheimers Dis. 2015. PMID: 25362040 Free PMC article.
References
-
- O’Riordan, K. J., Hu, N.-W. & Rowan, M. J. Aß facilitates LTD at Schaffer collateral synapses preferentially in the left hippocampus. Cell Rep.22, 2053–2065 (2018). - PubMed
-
- Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol.82, 239–259 (1991). - PubMed
-
- Moreno-Jiménez, E. P. et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med25, 554–560 (2019). - PubMed
-
- Hardy, J. & Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharm. Sci.12, 383–388 (1991). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

