An mRNA vaccine encoding five conserved Group A Streptococcus antigens
- PMID: 40592845
- PMCID: PMC12217327
- DOI: 10.1038/s41467-025-60580-0
An mRNA vaccine encoding five conserved Group A Streptococcus antigens
Abstract
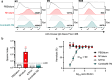
A commercial vaccine to address the high global burden of Group A Streptococcus (GAS) disease is an urgent and unmet medical need. Messenger RNA (mRNA) lipid-nanoparticle (LNP) vaccines represent a largely untapped platform for targeting bacterial pathogens. Here, we evaluate the immunogenicity and preclinical efficacy of a multicomponent mRNA-LNP vaccine formulation based on the GAS vaccine, Combo#5. Combo#5 mRNA-LNP antigens confer protection from infection in mouse intraperitoneal and subcutaneous challenge models. Combo#5 mRNA-LNP vaccination generates significantly increased frequencies and numbers of effector type CD4+ and CD8 + T cells in the spleen, enhances T follicular helper cells, germinal center B cells and memory B cells in the spleen and draining lymph nodes, and boosts the production of antigen-specific antibodies. These findings demonstrate the potential of the mRNA-LNP platform for the development of vaccines against bacterial pathogens.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors M.F., O.E., S.G., C.C., C.D. and O.P. were employed at Moderna TX during data acquisition and analysis and may hold stock or stock options. The authors N.H.P., I.S., R.A.B., A.J.C., I.G.C., S.H., R.P., J.R., L.D., B.P., J.N., G.E., D.M.P.D.O., B.F.C., N.B., M.A., S.B., G.T.B. and M.J.W. declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

