Enantioselective electroreductive alkyne-aldehyde coupling
- PMID: 40592859
- PMCID: PMC12219297
- DOI: 10.1038/s41467-025-60230-5
Enantioselective electroreductive alkyne-aldehyde coupling
Abstract
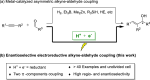
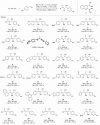
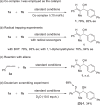
Electrocatalytic methods that facilitate the asymmetric reductive coupling of two π-components with complete control over regio-, stereo-, and enantioselectivity remain underexplored. Herein, we report a highly regio- and enantioselective cobaltaelectro-catalyzed alkyne-aldehyde coupling reaction, in which protons and electrons serve as the hydrogen source and reductant, respectively. Earth-abundant cobalt and air-stable (S,S)-2,3-bis(tert-butylmethylphosphino)quinoxaline (QuinoxP*) are used as the catalyst and ligand, respectively. A series of enantioenriched allylic alcohols can be constructed with excellent regio- (>19:1), stereo- (>19:1 E:Z), and enantioselectivity (up to 98% ee).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Gil, A., Albericio, F. & Álvarez, M. Role of the Nozaki–Hiyama–Takai–Kishi reaction in the synthesis of natural products. Chem. Rev.117, 8420–8446 (2017). - PubMed
-
- Butt, N. A. & Zhang, W. Transition metal-catalyzed allylic substitution reactions with unactivated allylic substrates. Chem. Soc. Rev.44, 7929–7967 (2015). - PubMed
-
- Godleski, S. A. in Comprehensive Organic Synthesis (eds Trost, Barry M. & Fleming, Ian) 585–661 (Pergamon, 1991).
-
- Lipshutz, B. H. & Sengupta, S. in Organic Reactions John Wiley & Sons, Inc. 135–631.
-
- Overman, L. E. Charge as a key component in reaction design. the invention of cationic cyclization reactions of importance in synthesis. Acc. Chem. Res.25, 352–359 (1992).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

