Increase in H5N1 vaccine antibodies confers cross-neutralization of highly pathogenic avian influenza H5N1
- PMID: 40592874
- PMCID: PMC12217246
- DOI: 10.1038/s41467-025-60714-4
Increase in H5N1 vaccine antibodies confers cross-neutralization of highly pathogenic avian influenza H5N1
Abstract
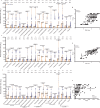
H5N1, a highly pathogenic avian influenza virus, presents pandemic risks due to its ability to adapt and spread among mammalian species. Vaccination may control its spread, but the effectiveness of existing H5N1 vaccines against circulating strains, especially clade 2.3.4.4b, remains uncertain. In this study, we assess neutralizing antibody responses to global circulating H5N1 strains, using sera from individuals vaccinated with an inactivated H5N1 vaccine (NCT00535665). Neutralization is measured against 17 pseudoviruses, representing circulating and vaccine H5 strains. Our results indicate that broad protective effects are observed only when high antibody titers are achieved by vaccination. Correlation analysis estimates that a pseudovirus-based neutralization titer of at least 1:980 is required to achieve a cross-protection rate above 60%. The findings suggest that the current H5N1 vaccine can elicit cross-neutralization of circulating H5N1 strains, if high antibody titers are achieved. Until updated H5N1 vaccines are developed, this vaccine may serve as a bridging measure.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

