Study on the adsorption of Pb2+ in aqueous solution by alkali modified wheat bran
- PMID: 40592876
- PMCID: PMC12216198
- DOI: 10.1038/s41598-025-03876-x
Study on the adsorption of Pb2+ in aqueous solution by alkali modified wheat bran
Abstract
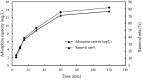
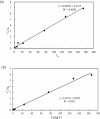
Due to human activities such as mining, metal smelting, and sewage irrigation, a significant amount of heavy metals have entered the environment, exacerbating the problem of heavy metal pollution in water bodies. The study of rapid adsorption and removal of lead is particularly critical due to its slow degradation and severe environmental impact. In this study, wheat bran was utilized as an adsorbent and modified using a sodium hydroxide solution. Kinetic and equilibrium adsorption studies were conducted to evaluate the adsorption performance of alkali modified wheat bran on lead ions in water. The adsorption behavior and mechanism of alkali modified wheat bran on Pb2+ were analyzed from three perspectives: time, concentration, and pH, in conjunction with surface functional groups and microscopic characteristics. The results indicated that the adsorption capacity of alkali modified wheat bran was achieved at pH 4, a concentration of 100 mg/L, and an duration of approximately 120 min. Experimental data shows that at about 120 min, its adsorption efficiency reaches its peak at 81.74%. The fitting results of the adsorption kinetics model and isothermal adsorption model indicate that the adsorption of modified wheat bran is characterized by single-layer chemical adsorption; The alkaline modification treatment increased the porosity of the surface pores of wheat bran, resulting in a rougher surface and an enhanced adsorption area. Furthermore, post-modification, the wheat bran exhibited increased C-H and C = O functional groups, thereby improving the adsorption of Pb2+.
Keywords: Adsorption; Alkali modification; Lead ion; Wheat Bran.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures






References
-
- Wang, X., Hu, X., Chen, Y. J. & Liu, Y. Adsorption and fixation of heavy metals in water environment by Biochar and modified Biochar. Environ. Eng.34, 32–37 (2016).
-
- Park, J. H., Choppala, G. K., Bolan, N. S., Chung, J. W. & Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant. Soil.348, 439–451 (2011). - DOI
-
- Wang, B. Y., Zhu, Y. E. & Li, H. Effects of vinegar residue Biochar on Pb (II) adsorption in water. Environ. Eng.34, 6–11 (2016).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

