Neuroprotection of photoreceptors by combined inhibition of both Fas and autophagy pathways in P23H mice
- PMID: 40592891
- PMCID: PMC12216854
- DOI: 10.1038/s41419-025-07793-9
Neuroprotection of photoreceptors by combined inhibition of both Fas and autophagy pathways in P23H mice
Abstract
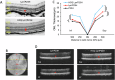
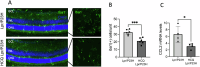
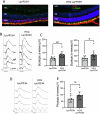
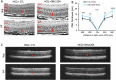
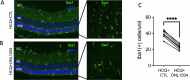
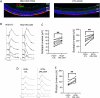
The P23H variant of rhodopsin (RHO) is a common cause of autosomal dominant retinitis pigmentosa (adRP). Our previous data have shown that both the Fas (CD95) death receptor and hyperactivation of autophagy contribute to photoreceptor (PR) death in a mouse model of P23H-RHO adRP. Individually, inhibition of Fas or suppression of autophagy flux improves PR survival and function. The purpose of this study is to examine whether combined inhibition of Fas receptor activation and reducing autophagy flux would have an additive effect on PR survival and function in the P23H mouse. We crossed the Lpr mouse (which contains a functional knockout of the Fas receptor) with the P23H mouse to generate the Lpr/P23H mouse. Hydroxychloroquine (HCQ) was given in the drinking water at P21 to reduce autophagy flux. As an alternative to genetic inhibition of the Fas receptor, pharmacological blockade of the Fas receptor was achieved using intravitreal injections of the Fas inhibitor, ONL1204, administered via intravitreal injection at P14 and 2 months of age. Fellow eyes were injected with vehicle solution as controls. PR cell death, structure and function of the retina, as well as the activation of immune cells, were evaluated. Consistent with previous data, the Lpr/P23H mice exhibited a decreased rate of photoreceptor degeneration and reduced inflammation compared with P23H. Treatment of these mice with HCQ further preserved photoreceptor survival and function lowered the activation of immune cells, and resulted in reduced production of inflammatory cytokines in the retina. These results were recapitulated in HCQ-treated P23H mice receiving intravitreal injections of ONL1204. Our data suggest that in the mouse model of P23H adRD, inhibition of both the Fas pathway and autophagy pathways results in a greater protective effect, demonstrating the potential multipronged therapeutic approach to reduce PR death and improve retinal function in patients with P23H.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Two of the authors have relevant disclosures. AJK is an employee of ONL Therapeutics, the company that produces ONL1204. DNZ is an employee of the University of Michigan and is also a co-founder of ONL Therapeutics. DNZ also holds patents through the University of Michigan that are licensed to ONL Therapeutics. DNZ laboratory receives grant funding from ONL Therapeutics. All other authors have no competing interests. Ethics approval: All animal experiments and experimental methods were performed in accordance with the guidelines set forth by the University of Michigan Animal Care Committee and conformed to the Use of Animals in Ophthalmology and Vision Research established by the Association for Research in Vision and Ophthalmology (ARVO).
Figures


References
-
- RetNet. https://sph.uth.edu/retnet/.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

