Phage-mediated peripheral kill-the-winner facilitates the maintenance of costly antibiotic resistance
- PMID: 40592899
- PMCID: PMC12219745
- DOI: 10.1038/s41467-025-61055-y
Phage-mediated peripheral kill-the-winner facilitates the maintenance of costly antibiotic resistance
Abstract
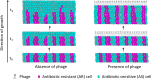
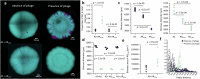
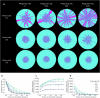
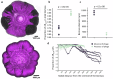
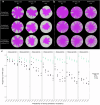
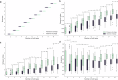
The persistence of antibiotic resistant (AR) bacteria in the absence of antibiotic pressure raises a paradox regarding the fitness costs associated with antibiotic resistance. These fitness costs should slow the growth of AR bacteria and cause them to be displaced by faster-growing antibiotic sensitive (AS) counterparts. Yet, even in the absence of antibiotic pressure, slower-growing AR bacteria can persist for prolonged periods of time. Here, we demonstrate a mechanism that can explain this apparent paradox. We hypothesize that lytic phage can modulate bacterial spatial organization to facilitate the persistence of slower-growing AR bacteria. Using surface-associated growth experiments with the bacterium Escherichia coli in conjunction with individual-based computational simulations, we show that phage disproportionately lyse the faster-growing AS counterpart cells located at the biomass periphery via a peripheral kill-the-winner dynamic. This enables the slower-growing AR cells to persist even when they are susceptible to the same phage. This phage-mediated selection is accompanied by enhanced bacterial diversity, further emphasizing the role of phage in shaping the assembly and evolution of bacterial systems. The mechanism is potentially relevant for any antibiotic resistance genetic determinant and has tangible implications for the management of bacterial populations via phage therapy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
The virulent bacteriophage Henu8 as an antimicrobial synergist against Escherichia coli.Microbiol Spectr. 2025 Jul;13(7):e0163324. doi: 10.1128/spectrum.01633-24. Epub 2025 May 16. Microbiol Spectr. 2025. PMID: 40377308 Free PMC article.
-
Antibiotic prophylaxis for preventing bacterial endocarditis following dental procedures.Cochrane Database Syst Rev. 2022 May 10;5(5):CD003813. doi: 10.1002/14651858.CD003813.pub5. Cochrane Database Syst Rev. 2022. PMID: 35536541 Free PMC article.
-
Evaluating the predictive power of combined gene expression dynamics from single cells on antibiotic survival.mSystems. 2025 Jun 17;10(6):e0158824. doi: 10.1128/msystems.01588-24. Epub 2025 May 20. mSystems. 2025. PMID: 40391890 Free PMC article.
-
Physicochemical, genomic, and phenotypic characterization of Escherichia phage BME3.Microbiol Spectr. 2025 Jul;13(7):e0130124. doi: 10.1128/spectrum.01301-24. Epub 2025 May 22. Microbiol Spectr. 2025. PMID: 40401929 Free PMC article.
-
Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev. 2017 Feb 9;2(2):CD003543. doi: 10.1002/14651858.CD003543.pub4. Cochrane Database Syst Rev. 2017. PMID: 28178770 Free PMC article.
References
-
- Seppälä, H. et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in Group A Streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N. Engl. J. Med.337, 441–446 (1997). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

