Stimulation of corticospinal neurons by optogenetic cAMP inductions promotes motor recovery after spinal cord injury in female rats via raphespinal tract modulation
- PMID: 40592902
- PMCID: PMC12216545
- DOI: 10.1038/s41467-025-61018-3
Stimulation of corticospinal neurons by optogenetic cAMP inductions promotes motor recovery after spinal cord injury in female rats via raphespinal tract modulation
Abstract
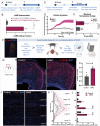
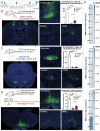
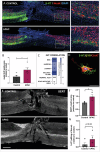
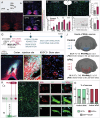
After spinal cord injury (SCI), cyclic adenosine monophosphate (cAMP) levels drop in the spinal cord, cortex and brainstem, unlike in regenerating peripheral neurons. To address SCI recovery, we expressed photoactivatable adenylate cyclase (bPAC) in corticospinal neurons of female rats with dorsal hemisection for on-demand cAMP inductions. bPAC stimulation restored passive and firing properties of corticospinal neurons, promoted early and sustained locomotor recovery and increased corticospinal tract plasticity. Additionally, bPAC enhanced sparing of lumbar-projecting brainstem neurons after SCI, accompanied by activation of cAMP signaling in the raphe-reticular formation and increased excitatory/inhibitory neurotransmitter balance. Accordingly, augmented density of serotonergic tracts was found caudal to the injury in bPAC rats, correlating with enhanced functional performance. Serotonergic implication in motor recovery was further evidenced by selective depletion, resulting in the abrogation of bPAC-mediated recovery. Overall, our findings underscore that cAMP induction in corticospinal neurons enhances locomotion after SCI, through a cortical rerouting pathway via the serotonergic descending tract.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- de la Torre-Valdovinos, B., L. P. Osuna-Carrasco, and C. A. C. Ramos, The Role of Supraspinal Structures for Recovery after SCI: From Motor Dysfunction to Mental Health, in Paraplegia. (2021).
-
- Van Wittenberghe, IC. & Peterson, D. C. Corticospinal Tract Lesion. (Treasure Island (FL): StatPearls Publishing (NBK542201, 2023). Available from: https://www.ncbi.nlm.nih.gov/books/NBK542201/. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

