Single-layer cluster ionic-chain networks with tetragonal pores
- PMID: 40592907
- PMCID: PMC12215711
- DOI: 10.1038/s41467-025-60879-y
Single-layer cluster ionic-chain networks with tetragonal pores
Abstract
Two-dimensional (2D) materials with intrinsic pores have attracted attention for catalytic and electronic applications. However, a significant gap exists between all-inorganic 2D networks with inorganic connectors and those with organic connectors due to the greater complexity of functionalizing inorganic molecules. Addressing this gap, we present a new class of 2D all-inorganic porous networks: single-layer cluster ionic-chain networks (CINs), constructed by using PW10M2 (M = Mn, Co) polyoxometalate (POM) clusters as nodes and end-capping agents for ionic chains. The integration of POM clusters into these networks significantly alters the electronic and band structures. Notably, the Mn-based CIN exhibits extremely high catalytic activity, achieving a toluene oxidation conversion rate of over 1.45 mmol g-1 h-1. Calculations suggest that POM clusters act as an 'electron buffer', stabilizing electron density at Mn sites and lowering the activation energy for toluene oxidation. This development showcases POM clusters as 'superatom' capping agents, establishing a pathway for all-inorganic 2D networks that could advance new catalytic materials with unique electronic properties.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

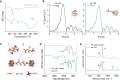

 ), PW10Mn2 clusters (
), PW10Mn2 clusters ( ), and Mn-CIN (
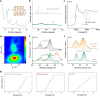
), and Mn-CIN ( ). Reaction conditions: 50 °C, 6 h. c The toluene conversion catalyzed by Mn-CIN at 50°C over 18 h. d Catalytic durability for Mn-CIN. Reaction conditions: 50 °C, 3 h. e The conversion rate of toluene oxidation for Mn-CIN at different temperatures for 3 h. f The toluene conversion rate of Mn-CIN compared with other reported systems for toluene oxidation.
). Reaction conditions: 50 °C, 6 h. c The toluene conversion catalyzed by Mn-CIN at 50°C over 18 h. d Catalytic durability for Mn-CIN. Reaction conditions: 50 °C, 3 h. e The conversion rate of toluene oxidation for Mn-CIN at different temperatures for 3 h. f The toluene conversion rate of Mn-CIN compared with other reported systems for toluene oxidation.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

