Prognostic model of lung adenocarcinoma from the perspective of cancer-associated fibroblasts using single-cell and bulk RNA-sequencing
- PMID: 40592920
- PMCID: PMC12216014
- DOI: 10.1038/s41598-025-05058-1
Prognostic model of lung adenocarcinoma from the perspective of cancer-associated fibroblasts using single-cell and bulk RNA-sequencing
Abstract
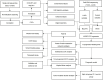
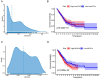
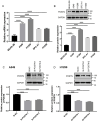
Cancer-associated fibroblasts (CAFs) play important roles in the progression of lung adenocarcinoma (LUAD). We examined CAF subgroups via gene ontology, pseudo-time, and cell communication analyses and explored their prognostic value in LUAD using a digital cytometric machine learning algorithm. Next, we got a prognostic model based on CAF subgroups. We also screened potential therapeutic target genes in LUAD and experimentally validated the proliferation, migration, and invasion phenotypes related to these target genes. We identified myofibroblastic CAFs (MyCAFs) and Immune-related CAFs (ImmCAFs) as the major CAF subgroups in LUAD. Further, our inverse convolution algorithm showed that MyCAFs have prognostic potential in LUAD, and via LASSO-COX model regression, we obtained a MyCAFs-related prognostic model. We found POSTN as a potential therapeutic target in LUAD. These findings serve as a foundation for further studies on CAFs.
Keywords: Cancer-associated fibroblasts; Lung adenocarcinoma; Machine learning; Single-cell and bulk RNA-sequencing.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures







Similar articles
-
Identification of a novel therapeutic candidate, NRK, in primary cancer-associated fibroblasts of lung adenocarcinoma microenvironment.J Cancer Res Clin Oncol. 2021 Apr;147(4):1049-1064. doi: 10.1007/s00432-020-03489-z. Epub 2021 Jan 2. J Cancer Res Clin Oncol. 2021. PMID: 33387038 Free PMC article.
-
Integration of Multi-Scale Profiling and Machine Learning Reveals the Prognostic Role of Extracellular Matrix-Related Cancer-Associated Fibroblasts in Lung Adenocarcinoma.Int J Med Sci. 2025 Jun 12;22(12):2956-2972. doi: 10.7150/ijms.113580. eCollection 2025. Int J Med Sci. 2025. PMID: 40657391 Free PMC article.
-
Single-cell RNA sequencing technology was employed to construct a risk prediction model for genes associated with pyroptosis and ferroptosis in lung adenocarcinoma.Respir Res. 2025 Jul 18;26(1):249. doi: 10.1186/s12931-025-03323-5. Respir Res. 2025. PMID: 40682067 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
References
-
- UyBico, S. J. et al. Lung cancer staging essentials: The new TNM staging system and potential imaging pitfalls. Radiographics30(5), 1163–1181. 10.1148/rg.305095166 (2010). - PubMed
-
- Thai, A. A., Solomon, B. J., Sequist, L. V., Gainor, J. F. & Heist, R. S. Lung cancer. Lancet398(10299), 535–554. 10.1016/S0140-6736(21)00312-3 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

