Associations between weight gain, integrase inhibitors antiretroviral agents, and gut microbiome in people living with HIV: a cross-sectional study
- PMID: 40592975
- PMCID: PMC12218934
- DOI: 10.1038/s41598-025-06500-0
Associations between weight gain, integrase inhibitors antiretroviral agents, and gut microbiome in people living with HIV: a cross-sectional study
Abstract
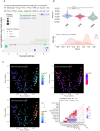
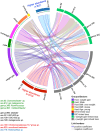
Dolutegravir and bictegravir are second-generation HIV integrase strand transfer inhibitors (INSTIs) that were previously associated with abnormal weight gain. This monocentric cross-sectional study investigates associations between weight gain during the first year after initiation of dolutegravir, bictegravir or other anchor drugs and gut microbiome diversity as well as taxa composition. The study enrolled 79 participants receiving dolutegravir, 32 receiving bictegravir and 10 receiving non-INSTI based regimens. Most of them were treatment experienced at initiation of those anchor drugs agents. Although weight gain was not linked to overall bacterial diversity, strong associations with specific taxa were demonstrated (FDR q < 0.01). Using multiple linear regression, we identified 4 distinct groups of bacteria associated with either dolutegravir, bictegravir, weight loss or weight gain under treatment, allowing a machine learning model to predict 15.9% of the weight gain variability regardless of sex, age and body mass index (RMSE: 0.0126). Dysosmobacter sp. and Haemophilus sp., two bacteria previously associated with host metabolism, were among the strongest predictors. Our findings link INSTIs, weight gain, and the gut microbiome. Future research should investigate the causal role of the identified taxa to improve our understanding of microbiome-drug interactions and further support personalized antiretroviral strategies.Trial registration: Eudra-CT 2020-001103-17 (registration date: 2020-12-01).
Trial registration: ClinicalTrials.gov NCT04805944.
Keywords: Dysosmobacter welbionis; Bictegravir; Dolutegravir; Microbiota; Tenofovir alafenamide; Weight gain.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study received approval by the local Ethical committee (Comité d’éthique Hospitalo-facultaire from Cliniques universitaires Saint-Luc-UCLouvain; 2020/30NOV/593) and regulatory authorities (Eudra-CT Number 2020-001103-17). All participants gave written informed consent before taking part in the study. All methods were performed in accordance with the relevant guidelines and regulations. Clinical trial: The trial is registered with ClinicalTrials.gov, NCT04805944 (registration date 2021–03-16). Competing interests: PDC is inventor on patent applications dealing with the use of specific bacteria and components in the treatment of different diseases. PDC was co-founder of The Akkermansia Company SA and Enterosys. The other authors declare no competing interests.
Figures

References
-
- Ambrosioni, J. et al. Major revision version 12.0 of the European AIDS Clinical Society guidelines 2023. HIV Med.24(11), 1126–1136 (2023). - PubMed
-
- WHO. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach 2021. Available from: https://www.who.int/publications/i/item/9789240031593 - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

