Topical application of the HSP90 inhibitor 17-AAG reduces skin inflammation and partially restores microbial balance: implications for atopic dermatitis therapy
- PMID: 40593012
- PMCID: PMC12216750
- DOI: 10.1038/s41598-025-05307-3
Topical application of the HSP90 inhibitor 17-AAG reduces skin inflammation and partially restores microbial balance: implications for atopic dermatitis therapy
Abstract
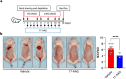
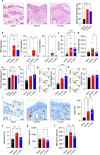
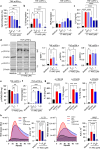
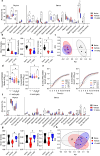
Heat shock proteins belonging to the HSP90 family promote inflammation and are potential therapeutic targets in inflammatory and autoimmune diseases. Here the effects of the HSP90 inhibitor 17-AAG applied topically were evaluated in a DNCB-induced murine model of atopic dermatitis (AD). The use of 17-AAG improved clinical disease activity without causing toxicity in the animals. Topical application of 17-AAG resulted in reduced epidermal hyperplasia, decreased expression of TSLP, IL-5, and IL-6, as well as reduced activation of NF-κB in the skin. In addition, the eosinophil proportion in the blood and eosinophil peroxidase (EPX) activity in the skin were significantly reduced in 17-AAG-treated AD mice. The inhibitory effects of 17-AAG on the production of epidermal alarmins, T-helper cell-associated cytokines, and ROS release were demonstrated in cultures of activated human keratinocytes, CD4+ T lymphocytes, and eosinophils, respectively. Finally, next-generation sequencing metagenomic approaches revealed that topical application of 17-AAG partially restored the normal gut microbiome in AD mice. Moreover, 17-AAG inhibited Staphylococcus aureus biofilm formation in vitro. The findings of this study, combined with the observed increase in HSP90 and EPX activity in the leukocytes of the analyzed cohort of AD patients, support the potential therapeutic use of HSP90 inhibitors in individuals with AD.
Keywords: Staphylococcus aureus; Eosinophiles; Heat shock proteins; Keratinocytes.; Microbiota; Mouse model.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: The study was conducted in accordance with the Declaration of Helsinki and was approved by the Independent Bioethics Committee for Scientific Research at the Medical University of Gdańsk, Poland (approval number NKBBN/209/2021). The animal study was reviewed and approved by the local authorities of the Animal Care and Use Committee in Bydgoszcz, Poland (approval number 53/2021).
Figures


Similar articles
-
Elephantopus scaber attenuates MC903-caused atopic dermatitis and decreases TNF-α/IFN-γ-induced chemokines by suppressing MKP-1-mediated AP-1 signaling in keratinocytes.J Ethnopharmacol. 2025 Aug 29;352:120267. doi: 10.1016/j.jep.2025.120267. Epub 2025 Jul 7. J Ethnopharmacol. 2025. PMID: 40633861
-
Nepetin Attenuates Atopic Dermatitis in HaCaT Cells and BALB/c Mice through MyD88-MKK3/6-Akt Signaling.Curr Med Chem. 2025;32(14):2775-2791. doi: 10.2174/0109298673244967231101114033. Curr Med Chem. 2025. PMID: 38310399
-
Taraxacum mongolicum Ameliorates DNCB-Induced Atopic Dermatitis-like Symptoms in Mice by Regulating Oxidative Stress, Inflammation, MAPK, and JAK/STAT/TSLP Signaling Pathways.Int J Mol Sci. 2025 Jul 9;26(14):6601. doi: 10.3390/ijms26146601. Int J Mol Sci. 2025. PMID: 40724851 Free PMC article.
-
Microbiome modulators for atopic eczema: a systematic review of experimental and investigational therapeutics.Expert Opin Investig Drugs. 2024 Apr;33(4):415-430. doi: 10.1080/13543784.2024.2326625. Epub 2024 Mar 6. Expert Opin Investig Drugs. 2024. PMID: 38441984
-
Cathelicidin expression in the pathogenesis of atopic dermatitis and the therapeutic potential of vitamin D.Nutr Res. 2025 Jul;139:113-123. doi: 10.1016/j.nutres.2025.05.006. Epub 2025 May 21. Nutr Res. 2025. PMID: 40517664 Review.
References
-
- Schopf, F. H., Biebl, M. M. & Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell. Biol.18, 345–360. 10.1038/nrm.2017.20 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

