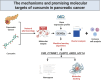
Unveiling the mechanisms and promising molecular targets of curcumin in pancreatic cancer through multi-dimensional data
- PMID: 40593037
- PMCID: PMC12217139
- DOI: 10.1038/s41598-025-05346-w
Unveiling the mechanisms and promising molecular targets of curcumin in pancreatic cancer through multi-dimensional data
Abstract
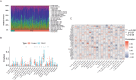
Pancreatic cancer (PC) is a highly aggressive and fatal malignancy, primarily affecting older males. Curcumin, a potential anti-cancer agent, has been shown to regulate key molecules in cancer progression, but its specific mechanisms in PC remain unclear. We conducted a comprehensive database search to identify curcumin-related targets in PC. Gene expression and immune correlations were analyzed using the GEO database, identifying differentially expressed hub genes (DEHGs). A method involving machine learning was employed to identify feature genes and create a nomogram, using external datasets and molecular docking for preliminary validation. Consensus clustering and subgroup comparisons were also performed based on DEHGs expression. We identified 35 DEHGs strongly associated with immune cell infiltration. Five feature genes (VIM, CTNNB1, CASP9, AREG, HIF1A) were used to build a nomogram, with the classification model showing AUC values above 0.9 in both training and validation groups. Molecular docking highlighted potential binding sites of five feature genes for curcumin. Clustering analysis categorized PC samples into four distinct subgroups: C1 and CII, which showed high expression and elevated immune cell infiltration, and C2 and CI, which exhibited the opposite pattern. Significant variations in scores of DEHG were seen between C1 and C2, in addition to between CI and CII. Curcumin may target DEHGs to influence PC, regulating immune and tumor proliferation mechanisms. These outcomes provide potential insights for medical applications and upcoming research.
Keywords: Curcumin; Machine learning; Network pharmacology; Nomogram; Pancreatic cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
A multi-phase approach using supervised algorithms and clinical models to generate high-accuracy signatures for pancreatic cancer.Comput Biol Med. 2025 Aug;194:110559. doi: 10.1016/j.compbiomed.2025.110559. Epub 2025 Jun 14. Comput Biol Med. 2025. PMID: 40517592
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Deciphering Shared Gene Signatures and Immune Infiltration Characteristics Between Gestational Diabetes Mellitus and Preeclampsia by Integrated Bioinformatics Analysis and Machine Learning.Reprod Sci. 2025 Jun;32(6):1886-1904. doi: 10.1007/s43032-025-01847-1. Epub 2025 May 15. Reprod Sci. 2025. PMID: 40374866
-
Molecular feature-based classification of retroperitoneal liposarcoma: a prospective cohort study.Elife. 2025 May 23;14:RP100887. doi: 10.7554/eLife.100887. Elife. 2025. PMID: 40407808 Free PMC article.
References
-
- Collisson, E. A., Bailey, P., Chang, D. K. & Biankin, A. V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol.16(4), 207–220. 10.1038/s41575-019-0109-y (2019). - PubMed
-
- Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71(3), 209–249. 10.3322/caac.21660 (2021). - PubMed
-
- Aggarwal, B. B., Sundaram, C., Malani, N. & Ichikawa, H. Curcumin: the Indian solid gold. Adv. Exp. Med. Biol.595, 1–75. 10.1007/978-0-387-46401-5_1 (2007). - PubMed
-
- Strimpakos, A. S. & Sharma, R. A. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal.10(3), 511–545. 10.1089/ars.2007.1769 (2008). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

