Impaired intestinal calcium absorption and osteopathy in ICR/Mlac-hydro mice with hypoparathyroidism and severe hydronephrosis
- PMID: 40593044
- PMCID: PMC12216024
- DOI: 10.1038/s41598-025-06485-w
Impaired intestinal calcium absorption and osteopathy in ICR/Mlac-hydro mice with hypoparathyroidism and severe hydronephrosis
Abstract
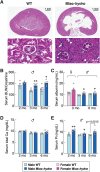
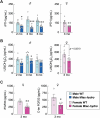
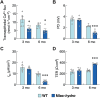
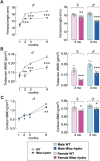
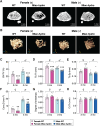
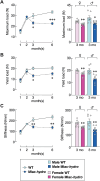
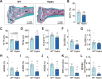
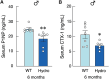
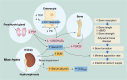
Abnormal fluid accumulation in the renal pelvis and calyces, with enlargement of the pelvicalyceal system, leads to a devastating disease known as hydronephrosis, which subsequently induces progressive renal impairment and mineral imbalance. Since the renal tubular cells play a role in the conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], an important calciotropic hormone, we hypothesized that the ICR/Mlac-hydro mice with bilateral non-obstructive hydronephrosis and hypoparathyroidism plausibly manifested derangements of calcium and bone metabolism. The results showed that Mlac-hydro mice had reductions in the levels of intact parathyroid hormone, 1,25(OH)2D3 and fibroblast growth factor-23, along with downregulated TRPV6 expression in the duodenum and ~ 50% reduction in calcium flux as determined by 45Ca radioactive tracer. Aberrant duodenal electrical properties, i.e., decreased potential difference and increased transepithelial resistance, were also observed, indicating reduced intestinal ion permeability. Both male and female Mlac-hydro mice had shorter femoral lengths and lower volumetric bone mineral density than wild-type mice. Ultra-high resolution micro-computed tomography further revealed defects in the trabecular bone microstructure, consistent with several abnormalities of bone histomorphometric parameters, e.g., reductions in osteoblast surface, active erosion surface, mineral apposition rate and bone formation rate. Bone mechanical properties, i.e., maximum load, yield load, and stiffness, were also impaired in both male and female Mlac-hydro mice, as evaluated by the three-point bending test. In conclusion, Mlac-hydro mice with hydronephrosis and hypoparathyroidism exhibited several features of calcium dysregulation and bone defects, e.g., impaired intestinal calcium absorption, poor bone mechanical properties, and low bone turnover, the latter of which suggested an association between adynamic bone disease and hydronephrosis. Our data, therefore, provide relevant information essential for the future development of drugs or treatments for hydronephrotic patients.
Keywords: Bone loss; Calcium absorption; Hydronephrosis; Micro-computed tomography (µCT); Vitamin D.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Chang, A. & Laszik, Z. G. in Robbins & Cotran pathologic basis of disease (eds V. Kumar, A.K. Abbas, & J.C. Aster) Ch. 20, 895–952 (Elsevier, 2021).
-
- Pain, V. M. & Strandhoy, J. W. in In Campbell-Walsh Urology. 1227–1273 (eds Assimis, D. G.) (Saunders Elsevier, 2007).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

