PGL-EXPO feasibility study of exposure to SDHi fungicides and risk of hereditary SDHx paraganglioma or pheochromocytoma
- PMID: 40593204
- PMCID: PMC12215505
- DOI: 10.1038/s41598-025-09166-w
PGL-EXPO feasibility study of exposure to SDHi fungicides and risk of hereditary SDHx paraganglioma or pheochromocytoma
Abstract
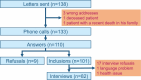
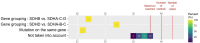
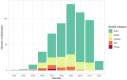
Paraganglioma and pheochromocytoma (PPGL) are rare neuroendocrine tumors with a strong genetic component. SDHx genes are well-known PPGL susceptibility genes. A role of environmental factors, such as succinate dehydrogenase inhibitor (SDHi) fungicides, is suspected in PPGL tumorigenesis. We evaluated the feasibility of a case-control study investigating the association between pesticides, focusing on SDHi fungicides, and the risk of SDHx-related PPGL. The study was conducted in a single referral center between March and June 2022. Cases were patients with PPGL and an SDHx germline mutation diagnosed between 2000 and 2021. Controls, were selected among relatives of cases, diagnosed as SDHx mutation carriers by a presymptomatic genetic test and without PPGL after tumor screening tests. A matching process was formulated to prioritize cases and controls that could be matched according to date of birth, type of gene mutated and distinct family. Phone interviews were used to collect data on general characteristics, PPGL risk factors, the domestic use of pesticides, residential history, occupational history. Addresses were geocoded. The presence of crops within a buffer of 1,500 m was estimated for residential addresses at diagnosis. The probability of occupational exposures to SDHi fungicides was assessed based on the French crop-exposure matrix PESTIMAT. An information letter was sent to 138 subjects out of the 193 followed-up by the center. The prioritization of recruitment was defined by the matching potential of each subject. Overall, 110 subjects were reached by phone, 101 accepted to be included, and 42 cases and 40 controls were interviewed (response rate: 81%). Missing data on domestic exposure to pesticides were more frequent for the households most back-in-time. Even for the oldest residential addresses, at least 81% of the addresses had a precise geocoding. In a buffer with a radius of 1500 m, arable land was present for 55% of subjects' addresses, vineyards and orchards for 5% and 3.8% respectively. Two subjects might have been exposed to SDHi fungicides in the occupational context. Our study showed high participation and response rates. Exposure to pesticides could be assessed with moderate to good precision, even for exposures far back in time.
Keywords: Case-control study; Feasibility study; Hereditary paraganglioma; SDH gene; SDHi fungicides.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Ethics and legal aspects: French law (in accordance with Articles L1122-1 and L1122-1-1 of the French Public Health Code) requires that participation in research of this nature (non-interventional epidemiological study) be offered to patients after they have been individually informed in writing, with the physician seeking the patient’s opposition to participation, and ensuring traceability of the absence of opposition (“non-objection form”). The law does not require written traceability to be directly ensured by the patient. These procedures have been implemented for this study (PGL.EXPO-1-ClinicalTrials.gov Identifier: NCT04481152) after approval by a committee for the protection of persons, in accordance with the law (Protection of Persons Committee of Ile-de-France X, Ref: 34-2020). It also received a favorable opinion from the French data protection authority, the Commission Nationale Informatique et Libertés (CNIL) (Ref: MLD/MFI/AR2114411). More specifically, before being included in the study, the patient receives a written information. The investigator orally explains to the patient the content of this information note, which consists of presenting the study, explaining its objectives and answering any questions the patient may have. The patient’s consent is given orally to the investigator, following the various items of information provided by the investigator, i.e. “informed consent”, who attests to this on the information note by indicating the following 4 items of information: patient’s surname/first name/identifier in the research; opposition expressed, date of issue of information, signature of person responsible for information.
Figures


Similar articles
-
Radiosurgical management of SDHx-related paraganglioma.J Clin Neurosci. 2025 Aug;138:111387. doi: 10.1016/j.jocn.2025.111387. Epub 2025 Jun 12. J Clin Neurosci. 2025. PMID: 40513256
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Psychological therapies for panic disorder with or without agoraphobia in adults: a network meta-analysis.Cochrane Database Syst Rev. 2016 Apr 13;4(4):CD011004. doi: 10.1002/14651858.CD011004.pub2. Cochrane Database Syst Rev. 2016. PMID: 27071857 Free PMC article.
References
-
- Berends, A. M. A. et al. Incidence of pheochromocytoma and sympathetic paraganglioma in the netherlands: A nationwide study and systematic review. Eur. J. Intern. Med.51, 68–73 (2018). - PubMed
-
- Leung, A. A., Hyrcza, M. D., Pasieka, J. L. & Kline, G. A. Incidence of pheochromocytoma and paraganglioma varies according to altitude: meta-regression analysis. Eur. J. Endocrinol.184, L21–L23 (2021). - PubMed
-
- Stenström, G. & Svårdsudd, K. Pheochromocytoma in Sweden 1958–1981: an analysis of the National Cancer registry data. Acta Med. Scand.220, 225–232 (1986). - PubMed
-
- Lenders, J. W. M. et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab.99, 1915–1942 (2014). - PubMed
MeSH terms
Substances
Grants and funding
- ANSES, N° 2022-CS-01_PPV22/Agence Nationale de Sécurité Sanitaire de l'Alimentation, de l'Environnement et du Travail
- ANSES, N° 2022-CS-01_PPV22/Agence Nationale de Sécurité Sanitaire de l'Alimentation, de l'Environnement et du Travail
- ANSES, N° 2022-CS-01_PPV22/Agence Nationale de Sécurité Sanitaire de l'Alimentation, de l'Environnement et du Travail
- ANSES, N° 2022-CS-01_PPV22/Agence Nationale de Sécurité Sanitaire de l'Alimentation, de l'Environnement et du Travail
- ANSES, N° 2022-CS-01_PPV22/Agence Nationale de Sécurité Sanitaire de l'Alimentation, de l'Environnement et du Travail
LinkOut - more resources
Full Text Sources
Medical

