TRBP modulates RLR signaling by inhibiting PKR-mediated antiviral stress granule formation
- PMID: 40593223
- PMCID: PMC12215557
- DOI: 10.1038/s41598-025-07121-3
TRBP modulates RLR signaling by inhibiting PKR-mediated antiviral stress granule formation
Abstract
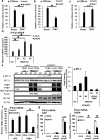
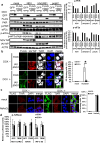
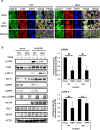
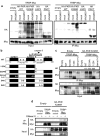
Stress granules (SGs) are dense aggregates of RNA and proteins that form in response to various cellular stresses. Virus-induced SGs, known as antiviral SGs (avSGs), play a crucial role in regulating retinoic acid-inducible gene I-like receptors (RLRs)-mediated antiviral innate immunity. However, the regulation of avSG formation remains not fully understood. In this study, we demonstrate that TAR-RNA binding protein (TRBP), an RNA silencing regulator, negatively regulates type I interferon (IFN) expression by inhibiting avSG formation in response to RNA virus infection. Overexpression of TRBP inhibits both IFN-β promoter activity and avSG formation following viral infection or the viral RNA mimic, polyinosinic-polycytidylic acid transfection. TRBP knockout cells exhibit enhanced phosphorylation and activation of IFN regulatory factor-3 (IRF-3) and increased IFN-β mRNA expression compared to wild-type cells. Additionally, depletion of G3BP1 and G3BP2, which are essential for SG formation, abolishes the inhibitory effect of TRBP on IRF-3 phosphorylation. Mechanistically, TRBP physically interacts with double-stranded RNA (dsRNA)-dependent protein kinase R (PKR), a key kinase involved in avSG formation, via its dsRNA-binding domains, and inhibits PKR activation. In summary, our findings reveal a novel function for TRBP as a negative regulator of RLR-mediated signaling through PKR-dependent inhibition of avSG formation.
Keywords: Antiviral innate immunity; IFN; RLR; Stress granule; TRBP.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
The SHFV nsp2 and nucleocapsid proteins recruit G3BP1 to sites of viral replication, but stress granules are not induced by the infection.J Virol. 2025 Jul 22;99(7):e0079425. doi: 10.1128/jvi.00794-25. Epub 2025 Jun 23. J Virol. 2025. PMID: 40548742 Free PMC article.
-
RNase L Amplifies Interferon Signaling by Inducing Protein Kinase R-Mediated Antiviral Stress Granules.J Virol. 2020 Jun 16;94(13):e00205-20. doi: 10.1128/JVI.00205-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295917 Free PMC article.
-
Glutamine modulates stress granule formation in cancer cells through core RNA-binding proteins.J Cell Sci. 2025 Jun 1;138(11):jcs263679. doi: 10.1242/jcs.263679. Epub 2025 Jun 6. J Cell Sci. 2025. PMID: 40376753 Free PMC article.
-
A closer look at mammalian antiviral condensates.Biochem Soc Trans. 2024 Jun 26;52(3):1393-1404. doi: 10.1042/BST20231296. Biochem Soc Trans. 2024. PMID: 38778761 Free PMC article. Review.
-
Application of stress granule core element G3BP1 in various diseases: A review.Int J Biol Macromol. 2024 Dec;282(Pt 5):137254. doi: 10.1016/j.ijbiomac.2024.137254. Epub 2024 Nov 6. Int J Biol Macromol. 2024. PMID: 39515684 Review.
References
-
- Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol.5, 730–737. 10.1038/ni1087 (2004). - PubMed
-
- Yoneyama, M. et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol.175, 2851–2858. 10.4049/jimmunol.175.5.2851 (2005). - PubMed
-
- Takahasi, K. et al. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol. Cell.29, 428–440. 10.1016/j.molcel.2007.11.028 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

