Nipah virus: pathogenesis, genome, diagnosis, and treatment
- PMID: 40593310
- PMCID: PMC12214056
- DOI: 10.1007/s00253-025-13474-6
Nipah virus: pathogenesis, genome, diagnosis, and treatment
Abstract
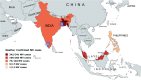
The highly infectious Nipah virus (NiV) is classified under the Paramyxoviridae family and is categorized under the genus Henipavirus. NiV spreads to humans through zoonotic transmission from reservoir host bats and other intermediate hosts. It is highly contagious and has a high case fatality rate (CFR) of ~ 40-80%. Only sporadic outbreaks have been reported so far, but like SARS-CoV2, NiV has a high pandemic potential and has been put on the World Health Organization (WHO) priority pathogen list. Currently, no clinically approved antivirals, immunotherapy, or vaccines are available to tackle NiV infection, thereby necessitating further research into its life cycle, transmission, and pathogenesis. This detailed review outlines the origin and spread of the Nipah virus, its modes of transmission, risk factors, its genome, key proteins, pathogenesis, and clinical features. We also discuss different diagnostic approaches and ongoing research to develop therapies ranging from antibodies to vaccines. KEY POINTS: •Pandemic preparedness for emerging and re-emerging viruses. •Novel approaches for diagnostics and therapeutics for Nipah viruse. •Global threat from biosafety level 4 pathogens. •Animal models for Nipah virus research.
Keywords: Anti-viral drugs; Clinical trials; Henipavirus; Nipah virus; Vaccines; Viral encephalitis; Viral outbreaks; WHO.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Not applicable. Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
The rising threat of Nipah virus: a highly contagious and deadly zoonotic pathogen.Virol J. 2025 May 10;22(1):139. doi: 10.1186/s12985-025-02728-4. Virol J. 2025. PMID: 40349023 Free PMC article. Review.
-
Laboratory Diagnosis of Hendra and Nipah: Two Emerging Zoonotic Diseases with One Health Significance.Viruses. 2025 Jul 17;17(7):1003. doi: 10.3390/v17071003. Viruses. 2025. PMID: 40733619 Free PMC article. Review.
-
Antigenic and mutational insights into the Nipah virus G glycoprotein: implications for viral entry, host specificity, therapeutics, and vaccine development.PeerJ. 2025 Aug 12;13:e19835. doi: 10.7717/peerj.19835. eCollection 2025. PeerJ. 2025. PMID: 40852381 Free PMC article. Review.
-
Detection of Nipah Virus in Human Milk: A Novel Finding.J Med Virol. 2025 Jul;97(7):e70445. doi: 10.1002/jmv.70445. J Med Virol. 2025. PMID: 40586678
-
Serologic Evidence of Human Exposure to Bat-Borne Zoonotic Paramyxoviruses, Cambodia.Viruses. 2025 Aug 21;17(8):1146. doi: 10.3390/v17081146. Viruses. 2025. PMID: 40872860 Free PMC article.
References
-
- Abdullah S, Tan CT (2014) Henipavirus encephalitis. Handb Clin Neurol 123:663–670. 10.1016/B978-0-444-53488-0.00032-8 - PubMed
-
- Aguilar HC, Matreyek KA, Filone CM, Hashimi ST, Levroney EL, Negrete OA, Bertolotti-Ciarlet A, Choi DY, McHardy I, Fulcher JA, Su SV, Wolf MC, Kohatsu L, Baum LG, Lee B (2006) N-glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J Virol 80:4878–4889. 10.1128/JVI.80.10.4878-4889.2006 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

