Investigating Tumour Responses to Combinations of Radiotherapy and Hyperthermia
- PMID: 40593385
- PMCID: PMC12213896
- DOI: 10.1007/s11538-025-01449-7
Investigating Tumour Responses to Combinations of Radiotherapy and Hyperthermia
Abstract
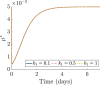
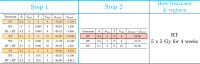
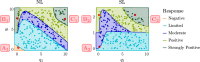
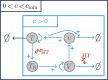
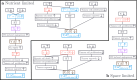
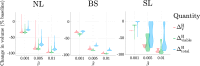
Hyperthermia (HT) is a promising candidate for enhancing the efficacy of radiotherapy (RT), but its use in the clinic has been limited by incomplete understanding of its interactions with RT. In this work, we investigate tumour responses to high temperature HT alone and combined with RT, focussing on how two different mechanisms for growth control may impact tumour sensitivity to these treatments. We extend an existing ordinary differential equation model of tumour growth and RT response to include high HT. In the absence of treatment, this model distinguishes between growth arrest due to nutrient insufficiency and competition for space, and exhibits three growth regimes: nutrient limited (NL), space limited (SL) and bistable (BS), where both mechanisms for growth arrest coexist. We construct three virtual tumour populations corresponding to the NL, SL and BS regimes and, for each population, we identify the treatment (RT, HT or RT + HT) and dosing regimen that maximise the reduction in tumour burden at the treatment end-point. We thus generate experimentally testable predictions that may explain highly variable experimental and clinical responses to RT and HT and assist patient-specific treatment design.
Keywords: Growth arrest mechanisms; Hyperthermia; ODE system; Radiotherapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: We declare we have no conflict of interest.
Figures













References
-
- Attar MM, Haghpanahi M, Amanpour S et al (2014) Analysis of bioheat transfer equation for hyperthermia cancer treatment. J Mech Sci Technol 28(2):763–771. 10.1007/s12206-013-1141-4 - DOI
-
- Bosque JJ, Calvo GF, Navarro MC (2022) Modelling the effect of vascular status on tumour evolution and outcome after thermal therapy. Appl Math Model 110:207–240. 10.1016/j.apm.2022.05.029 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

