AK4 promotes nasopharyngeal carcinoma metastasis and chemoresistance by activating NLRP3 inflammatory complex
- PMID: 40593461
- PMCID: PMC12217281
- DOI: 10.1038/s41419-025-07805-8
AK4 promotes nasopharyngeal carcinoma metastasis and chemoresistance by activating NLRP3 inflammatory complex
Abstract
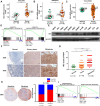
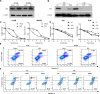
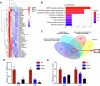
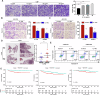
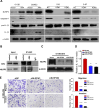
Metastasis is the main cause of treatment failure in nasopharyngeal carcinoma (NPC). Our previous study developed a transcriptomics-based gene signature (AK4, CPAMD8, DDAH1, and CRTR1) to predict metastasis in NPC and identify candidates that could benefit from induction chemotherapy (IC). Of these, adenylate kinase 4 (AK4) is a potent oncogene involved in the malignant progression of a variety of tumors. This study investigated the expression and mechanism of action of AK4, a member of the AK family of enzymes, in NPC. Quantitative real-time PCR, western blotting, and immunohistochemistry revealed that AK4 was upregulated in NPC and correlated with metastasis and chemoresistance. Stable ectopic overexpression of AK4 in NPC cell lines conferred resistance to taxol-induced apoptosis, promoted the migration, invasion, and EMT phenotype, and induced IL-1β secretion by activating the NLRP3 signaling pathway; knockdown of AK4 had the opposite effects. Mechanistically, AK4 co-localized with NNT, upregulated NLRP3 and IL-1β, and consequently altered NPC cell metastasis and chemoresistance. AK4 may play a role in the development of NPC and represent a potential therapeutic target.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All animal experiments were conducted according to the protocol approved by the Animal Care and Use Committee of Sun Yat-sen University Cancer Center. This study was conducted in accordance with the guidelines of the Institute Research Ethics Committee of Sun Yat-sen University Cancer Center.
Figures



References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. - PubMed
-
- Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer. 2010;29:517–26. - PubMed
-
- Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–8. - PubMed
-
- Liu LT, Tang LQ, Chen QY, Zhang L, Guo SS, Guo L, et al. The prognostic value of plasma Epstein-Barr Viral DNA and tumor response to neoadjuvant chemotherapy in advanced-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2015;93:862–9. - PubMed
-
- Tang XR, Li YQ, Liang SB, Jiang W, Liu F, Ge WX, et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study. Lancet Oncol. 2018;19:382–93. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

