Serum multi-trace elements and post-stroke cognitive impairment: a prospective observational cohort study
- PMID: 40593472
- PMCID: PMC12216882
- DOI: 10.1038/s41398-025-03420-5
Serum multi-trace elements and post-stroke cognitive impairment: a prospective observational cohort study
Abstract
Post-stroke cognitive impairment (PSCI) significantly affects stroke survivors. Identifying modifiable risk factors for PSCI is essential. Serum multi-trace elements are crucial for neurological function but vary in concentration among older adults. It remains unclear whether increasing multi-trace elements can reduce the incidence of PSCI. We investigated the associations between baseline serum multi-trace elements and PSCI. The Montreal Cognitive Assessment defined PSCI. We used logistic regression analyses to evaluate the association between serum multi-trace elements and PSCI. Subsequently, we assessed the associations between serum multi-trace elements and three different cognitive domains using the Kruskal-Wallis test. We further evaluated improvements in the predictive ability of serum multi-trace elements. Finally, 626 patients (mean age: 62.85 ± 7.54 years) were followed up for a median of 1.2 years. Lower concentrations of serum iron (odds ratio [OR] = 2.498, 95% confidence interval [CI]: 1.505-4.145) and zinc (OR = 2.015, 95% CI: 1.233-3.293) were associated with a higher PSCI risk. Higher concentrations of serum iron (OR = 0.368, 95% CI: 0.227-0.595) and magnesium (OR = 0.273, 95% CI: 0.164-0.454), along with lower concentrations of serum copper (OR = 0.544, 95% CI: 0.34-0.872), were significantly correlated with a lower PSCI risk. Cognitive impairments varied across multi-trace elements. Serum iron affected wider cognition, while magnesium and copper levels were strongly associated with language and executive function. Adding serum multi-trace elements to the conventional model improved PSCI risk reclassification (area under curve: 0.676-0.718). Multi-trace elements may influence PSCI progression. This study was registered with the Chinese Clinical Trial Registry (URL: https://www.chictr.org.cn/ ; unique identifier: ChiCTR1900022675).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: The Ethics Committee of the First Hospital of Jilin University approved the study protocol. Written informed consent was obtained from all the participants. All methods were performed in accordance with the relevant guidelines and regulations.
Figures
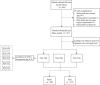


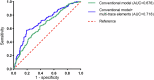
References
-
- Wang K, Dong Q, Yu J, Hu W. Expert consensus on post-stroke cognitive impairment. Chin J Stroke. 2021;16:376–89.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

