Cost-effectiveness of the ReDIRECT/counterweight-plus weight management programme to alleviate symptoms of long COVID
- PMID: 40593486
- PMCID: PMC12218384
- DOI: 10.1038/s41467-025-59909-6
Cost-effectiveness of the ReDIRECT/counterweight-plus weight management programme to alleviate symptoms of long COVID
Abstract
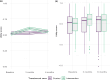
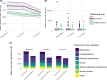
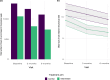
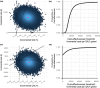
Long-term effects of COVID-19 infection, termed Long COVID (LC), are associated with reduced quality of life. Symptoms associated with overweight/obesity overlap with and may aggravate those of LC. This paper reports the economic evaluation alongside the ReDIRECT Trial, which evaluated the impact of an evidence-based, remotely-delivered weight management programme on self-reported symptoms of LC in those living with overweight/obesity in the United Kingdom. Recruited participants (n = 234) were randomly allocated to the intervention group (weight management) or control group (usual care). Incremental costs and Quality-Adjusted Life Years (QALYs) were calculated using intervention cost, healthcare resource use and EQ-5D-5L data collected at baseline, three and 6 months. In this work, we show that the ReDIRECT intervention is likely cost-effective in improving LC symptoms from an NHS/PSS perspective, compared to usual care (Incremental Cost-Effectiveness Ratio of £14,754/QALY). Adopting a broader societal perspective, the intervention becomes potentially cost saving compared to usual care.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: N.B. is an employee and shareholder of Counterweight Ltd., subcontracted to the University of Glasgow to deliver the ReDIRECT intervention. A.M. is a member of Clinical Steering Committee for ARC Medical Inc. N.S. has received institutional grant support from AstraZeneca, Boehringer Ingelheim, Novartis, Roche Diagnostics and honoraria from Abbott Laboratories, AbbVie, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, Menarini-Ricerche, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi. M.L. has received lecturing fees from Novo Nordisk, Lilly, Nestle, Oviva, Merck, and Sanofi and is a medical advisor to Counterweight Ltd, with fees paid to the University of Glasgow. The remaining authors declare no competing interests.
Figures
References
-
- Haag, L. et al. The remote diet intervention to reduce Long COVID symptoms trial (ReDIRECT): protocol for a randomised controlled trial to determine the effectiveness and cost-effectiveness of a remotely delivered supported weight management programme for people with Long COVID and excess weight, with personalised improvement goals [version 2; peer review: 2 approved]. NIHR Open Res.2, 57 (2023). - PMC - PubMed
-
- NICE. COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE Guidance. https://www.nice.org.uk/guidance/ng188/chapter/5-Management (2020). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

