A heterozygous CEBPA mutation disrupting the bZIP domain in a RUNX1 and SRSF2 mutational background causes MDS disease progression
- PMID: 40593493
- PMCID: PMC12219322
- DOI: 10.1038/s41467-025-60192-8
A heterozygous CEBPA mutation disrupting the bZIP domain in a RUNX1 and SRSF2 mutational background causes MDS disease progression
Abstract
Myelodysplastic syndrome disease (MDS) is caused by the successive acquisition of mutations and thus displays a variable risk for progression to AML. Mutations in CEBPA are commonly associated with a high risk of disease progression, but whether they are causative for AML development is unclear. To analyse the molecular basis of disease progression we generated MDS patient-derived induced pluripotent stem cells from a low risk male patient harbouring RUNX1/SRSF2 mutations. This experimental model faithfully recapitulates the patient disease phenotypes upon hematopoietic differentiation. Introduction of a frameshift mutation affecting the C/EBPα bZIP domain in cells from low-risk stages mimicks disease progression by reducing clonogenicity of myeloid cells, blocking granulopoiesis and increasing erythroid progenitor self-renewal capacity. The acquisition of this mutation reshapes the chromatin landscape at distal cis-regulatory regions and promotes changes in cellular composition as observed by single cell RNAseq. Mutant C/EBPα is therefore causative for MDS disease progression. Our work identifies mutant CEBPA as causative for MDS disease progression, providing a new isogenic MDS experimental model for drug screening to improve diagnostic and therapeutic strategies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Pablo Menendez is co-founder of OneChain Immunotherapeutics, a spin-off company from the Josep Carreras Leukaemia Institute. The remaining authors declare no competing interests.
Figures


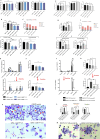




References
-
- Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood127, 2391–2405 (2016). - PubMed
MeSH terms
Substances
Grants and funding
- CC2027/WT_/Wellcome Trust/United Kingdom
- 15001/Bloodwise
- PI21/01641/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- MR/S021469/1/RCUK | MRC | Medical Research Foundation
- PI19/00730/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

