Enhanced role of the entorhinal cortex in adapting to increased working memory load
- PMID: 40593531
- PMCID: PMC12217308
- DOI: 10.1038/s41467-025-60681-w
Enhanced role of the entorhinal cortex in adapting to increased working memory load
Abstract
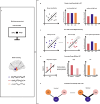
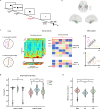
In daily life, we frequently encounter varying demands on working memory (WM), yet how the brain adapts to high WM load remains unclear. To address this question, we recorded intracranial EEG from hippocampus, entorhinal cortex (EC), and lateral temporal cortex (LTC) in humans performing a task with varying WM loads (load 4, 6, and 8). Using multivariate machine learning analysis, we decoded WM load using the power from each region as neural features. The results showed that the EC exhibited both higher decoding accuracy on medium-to-high load and superior cross-regional generalization. Further analysis revealed that removing EC-related information significantly reduced residual decoding accuracy in the hippocampus and LTC. Additionally, we found that WM maintenance was associated with enhanced phase synchronization between the EC and other regions. This inter-regional communication increased as WM load rose. These results suggest that under higher WM load, the brain relies more on the EC, a key connector that links and shares information with the hippocampus and LTC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Grants and funding
- 32271085/National Natural Science Foundation of China (National Science Foundation of China)
- 32400883/National Natural Science Foundation of China (National Science Foundation of China)
- 5244049/Natural Science Foundation of Beijing Municipality (Beijing Natural Science Foundation)
- 204651/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources

