Host glutathione is required for Rickettsia parkeri cell division and intracellular survival
- PMID: 40593553
- PMCID: PMC12216324
- DOI: 10.1038/s41467-025-60509-7
Host glutathione is required for Rickettsia parkeri cell division and intracellular survival
Abstract
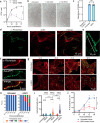
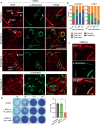
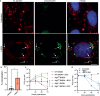
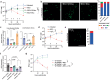
Intracellular bacteria rely on eukaryotic metabolites for their fitness and pathogenesis. Yet, the mechanisms of how host metabolites promote bacterial physiology and immune evasion are often unclear. Here, we employed obligate cytosolic Rickettsia parkeri, which parasitizes over fifty host metabolites, to investigate bacterial utilization of host glutathione (GSH). We observed that GSH depletion impaired R. parkeri intracellular survival. Super-resolution microscopy revealed that GSH depletion caused bacterial chaining in the host cytosol, prohibiting proper actin-based motility and cell-to-cell spread. GSH was especially critical for bacterial survival in primary macrophages, where it enabled R. parkeri to evade ubiquitylation and antibacterial autophagy. Cell division and survival defects were restored by supplementing N-acetylcysteine, suggesting that GSH serves as a cysteine source for R. parkeri. Together, these data suggest that Rickettsia requires GSH as a nutrient source to promote cell division, actin-based motility, evasion of antibacterial autophagy, and intracellular survival. This knowledge contributes to the expanding paradigm that GSH plays diverse roles in the pathogenesis of intracellular bacteria and represents a potential target for host-acting therapeutics.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Biggs, H. M. et al. Diagnosis and management of tickborne rickettsial diseases: rocky mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis-United States. Mortal. Wkly. Rep.65, 1–44 (2016). - PubMed
-
- Dantas-Torres, F. Rocky Mountain spotted fever. Lancet Infect. Dis.7, 724–732 (2007). - PubMed
-
- Kirkland, K. B., Wilkinson, W. E. & Sexton, D. J. Therapeutic delay and mortality in cases of Rocky Mountain spotted fever. Clin. Infect. Dis.20, 1118–1121 (1995). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

