Light-induced structural adaptation of the bundle-shaped phycobilisome from thylakoid-lacking cyanobacterium Gloeobacter violaceus
- PMID: 40593595
- PMCID: PMC12216592
- DOI: 10.1038/s41467-025-60673-w
Light-induced structural adaptation of the bundle-shaped phycobilisome from thylakoid-lacking cyanobacterium Gloeobacter violaceus
Abstract
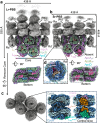
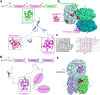
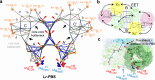
Gloeobacter diverged from other lineages early in cyanobacterial evolution, preferentially growing under low light intensity conditions. Among cyanobacteria, G. violaceus exhibits unique features, including lack of a thylakoid membrane and bundle-shaped antenna phycobilisomes (PBSs), densely packed and well-organized on the plasma membrane. However, without high-resolution structures, it has remained unclear how G. violaceus PBSs assemble into a bundle-shaped configuration. Here we solve the cryo-EM structures of PBSs from G. violaceus cells cultured under low (Sr-PBS) or moderate (Lr-PBS) light intensity. These structures reveal two unique linker proteins, LRC91kDa and LRC81kDa, that play a key role in the PBS architecture. Analysis of the bilin arrangement indicates that the bundle-shaped structure allows efficient energy transfer among rods. Moreover, comparison between Lr-PBS and Sr-PBS uncovers a distinct mode of adaption to increased light intensity wherein the ApcA2-ApcB3-ApcD layer can be blocked from binding to the core by altering structural elements exclusively found in the G. violaceus LCM. This study illustrates previously unrecognized mechanisms of assembly and adaptation to varying light intensity in the bundle-shaped PBS of G. violaceus.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Functional Modification of Cyanobacterial Phycobiliprotein and Phycobilisomes through Bilin Metabolism Control.ACS Synth Biol. 2024 Aug 16;13(8):2391-2401. doi: 10.1021/acssynbio.4c00094. Epub 2024 Jul 22. ACS Synth Biol. 2024. PMID: 39038807 Free PMC article.
-
Phycobilisomes from the mutant cyanobacterium Synechocystis sp. PCC 6803 missing chromophore domain of ApcE.Biochim Biophys Acta Bioenerg. 2018 Apr;1859(4):280-291. doi: 10.1016/j.bbabio.2018.01.003. Epub 2018 Jan 31. Biochim Biophys Acta Bioenerg. 2018. PMID: 29391123
-
Mutagenic analysis of the bundle-shaped phycobilisome from Gloeobacter violaceus.Photosynth Res. 2023 Nov;158(2):81-90. doi: 10.1007/s11120-023-01003-3. Epub 2023 Feb 27. Photosynth Res. 2023. PMID: 36847892
-
The Structure and Mechanism of Energy Transfer in Phycobilisomes.Annu Rev Microbiol. 2025 Jul 29. doi: 10.1146/annurev-micro-051024-074722. Online ahead of print. Annu Rev Microbiol. 2025. PMID: 40729612 Review.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
References
-
- Nelissen, B., Van De Peer, Y., Wilmotte, A. & De Wachter, R. An early origin of plastids within the cyanobacterial divergence is suggested by evolutionary trees based on complete 16S rRNA sequences. Mol. Biol. Evol.12, 1166–1173 (1995). - PubMed
-
- Blank, C. E. & Sanchez-Baracaldo, P. Timing of morphological and ecological innovations in the cyanobacteria—a key to understanding the rise in atmospheric oxygen. Geobiology8, 1–23 (2010). - PubMed
-
- Sanchez-Baracaldo, P., Hayes, P. K. & Blank, C. E. Morphological and habitat evolution in the cyanobacteria using a compartmentalization approach. Geobiology3, 145–165 (2005).
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

