HDL-bound S1P affects the subventricular niche and early neuropathological features of Alzheimer's disease
- PMID: 40593621
- PMCID: PMC12216268
- DOI: 10.1038/s41467-025-60750-0
HDL-bound S1P affects the subventricular niche and early neuropathological features of Alzheimer's disease
Abstract
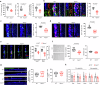
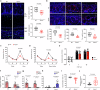
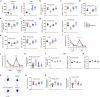
Circulating blood factors are critical for homeostasis of the adult ventricular-subventricular (V-SVZ) and subgranular zones, which contain neural stem cells (NSCs) crucial for sustained neurogenesis. Circulating sphingosine-1-phosphate (S1P) bound to apolipoprotein M (ApoM), a principal component of high-density lipoproteins, is involved in various biological processes, but its role in neurogenic niches is poorly understood. Herein, using Apom-/- mice, we show that blood ApoM-S1P deficiency impairs the SVZ-NSC pool, neurogenesis, ependymal cell polarity, and cerebrospinal fluid flow, leading to olfactory dysfunction and ventricular enlargement, early neuropathological features of Alzheimer's disease (AD). Enhancing the complex significantly rescues these defects by activating S1P1 receptor signaling in SVZ-NSCs. Consistently, blood ApoM-S1P levels are reduced in early AD patients and correlate with olfactory deficits and ventricular enlargement. Similar abnormalities are recapitulated in young APP/PS1 mice and reversed by restoring blood ApoM-S1P levels. Thus, these data reveal pathogenic mechanisms underlying early neuropathological features of AD and identify the blood ApoM-S1P complex as a potential diagnostic and therapeutic target.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

