The virulence regulator CovR boosts CRISPR-Cas9 immunity in Group B Streptococcus
- PMID: 40593653
- PMCID: PMC12216829
- DOI: 10.1038/s41467-025-60871-6
The virulence regulator CovR boosts CRISPR-Cas9 immunity in Group B Streptococcus
Abstract
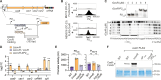
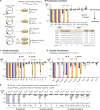
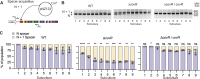
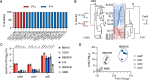
CRISPR-Cas9 immune systems protect bacteria from foreign DNA. However, immune efficiency is constrained by Cas9 off-target cleavages and toxicity. How bacteria regulate Cas9 to maximize protection while preventing autoimmunity is not understood. Here, we show that the master regulator of virulence, CovR, regulates CRISPR-Cas9 immunity against mobile genetic elements in Streptococcus agalactiae, a pathobiont responsible for invasive neonatal infections. We show that CovR binds to and represses a distal promoter of the cas operon, integrating immunity within the virulence regulatory network. The CovR-regulated promoter provides a controlled increase in off-target cleavages to counteract mutations in the target DNA, restores the potency of old immune memory, and stimulates the acquisition of new memory in response to recent infections. Regulation of Cas9 by CovR is conserved at the species level, with lineage specificities suggesting different adaptive trajectories. Altogether, we describe the coordinated regulation of immunity and virulence that enhances the bacterial immune repertoire during host-pathogen interaction.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

