Noncanonical short-latency auditory pathway directly activates deep cortical layers
- PMID: 40593664
- PMCID: PMC12216178
- DOI: 10.1038/s41467-025-61020-9
Noncanonical short-latency auditory pathway directly activates deep cortical layers
Abstract
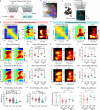
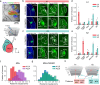
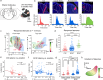
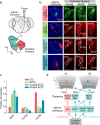
Auditory processing in the cerebral cortex is considered to begin with thalamocortical inputs to layer 4 (L4) of the primary auditory cortex (A1). In this canonical model, A1 L4 inputs initiate a hierarchical cascade that propagates to higher-order cortices for slower integration of complex sounds. Here, we identify parallel ascending pathways in mice that bypass A1 and directly reach the secondary auditory cortex (A2), alongside the canonical hierarchical route. We found that layer 6 (L6) of both A1 and A2 receive short-latency (<10 ms) sound inputs via higher-order thalamic nuclei. Additionally, A2 L4 is innervated by a caudal subdivision of the traditionally defined primary thalamus, which we now re-classify as non-primary. Notably, both identified thalamic regions receive projections from distinct subdivisions of the higher-order inferior colliculus, which in turn receive direct projections from cochlear nucleus neurons. Thus, higher-order auditory cortex integrates both slower, pre-processed information and rapid, direct sensory inputs, enabling parallel processing of fast sensory information across cortical areas.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Update of
-
Noncanonical Short-Latency Auditory Pathway Directly Activates Deep Cortical Layers.bioRxiv [Preprint]. 2025 Feb 11:2025.01.06.631598. doi: 10.1101/2025.01.06.631598. bioRxiv. 2025. Update in: Nat Commun. 2025 Jul 1;16(1):5911. doi: 10.1038/s41467-025-61020-9. PMID: 39829930 Free PMC article. Updated. Preprint.
Similar articles
-
Noncanonical Short-Latency Auditory Pathway Directly Activates Deep Cortical Layers.bioRxiv [Preprint]. 2025 Feb 11:2025.01.06.631598. doi: 10.1101/2025.01.06.631598. bioRxiv. 2025. Update in: Nat Commun. 2025 Jul 1;16(1):5911. doi: 10.1038/s41467-025-61020-9. PMID: 39829930 Free PMC article. Updated. Preprint.
-
Neural Correlates of Perceptual Plasticity in the Auditory Midbrain and Thalamus.J Neurosci. 2025 Mar 5;45(10):e0691242024. doi: 10.1523/JNEUROSCI.0691-24.2024. J Neurosci. 2025. PMID: 39753303
-
A parallel tonotopically arranged thalamocortical circuit for sound processing.Neuron. 2025 Jun 18;113(12):1998-2013.e6. doi: 10.1016/j.neuron.2025.03.022. Epub 2025 Apr 15. Neuron. 2025. PMID: 40239654
-
Auditory integration training and other sound therapies for autism spectrum disorders (ASD).Cochrane Database Syst Rev. 2011 Dec 7;2011(12):CD003681. doi: 10.1002/14651858.CD003681.pub3. Cochrane Database Syst Rev. 2011. PMID: 22161380 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Beltramo, R. & Scanziani, M. A collicular visual cortex: neocortical space for an ancient midbrain visual structure. Science363, 64–69 (2019). - PubMed
-
- Mo, C. et al. General Organization and Parallel Pathways in the Somatosensory System. in The Cerebral Cortex and Thalamus (eds. Usrey, M. W. & Sherman, M. S.) 258–268 (Oxford University PressNew York, 2023).
MeSH terms
Grants and funding
- P50 HD103573/HD/NICHD NIH HHS/United States
- F31 NS111849/NS/NINDS NIH HHS/United States
- P30 NS045892/NS/NINDS NIH HHS/United States
- RF1 NS128873/NS/NINDS NIH HHS/United States
- RF1NS128873/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 DC017516/DC/NIDCD NIH HHS/United States
- F31-NS111849/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01DC017516/U.S. Department of Health & Human Services | NIH | National Institute on Deafness and Other Communication Disorders (NIDCD)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

