Mosquito immune cells enhance dengue and Zika virus infection in Aedes aegypti
- PMID: 40593674
- PMCID: PMC12214649
- DOI: 10.1038/s41467-025-61139-9
Mosquito immune cells enhance dengue and Zika virus infection in Aedes aegypti
Abstract
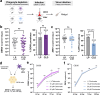
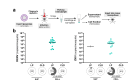
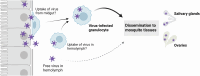
Mosquito-borne arboviruses cause more than 400 million annual infections, yet despite their public health importance, the mechanisms by which arboviruses infect and disseminate in the mosquito host are not well understood. Here, we provide evidence that dengue virus and Zika virus actively infect Aedes aegypti hemocytes and demonstrate, through phagocyte depletion, that hemocytes facilitate virus infection to peripheral tissues including the ovaries and salivary glands. Adoptive transfer experiments further reveal that virus-infected hemocytes efficiently confer virus infection to naïve recipient mosquitoes. Together, these data support a model of arbovirus dissemination where infected hemocytes enhance virus infection of mosquito tissues required for transmission, which parallels vertebrate systems where immune cell populations promote virus dissemination. This study significantly advances our understanding of virus infection dynamics in the mosquito host and highlights potential conserved roles of immune cells in arbovirus infection across vertebrate and invertebrate systems.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Update of
-
Mosquito immune cells enhance dengue and Zika virus dissemination in Aedes aegypti.bioRxiv [Preprint]. 2024 Apr 4:2024.04.03.587950. doi: 10.1101/2024.04.03.587950. bioRxiv. 2024. Update in: Nat Commun. 2025 Jul 1;16(1):5891. doi: 10.1038/s41467-025-61139-9. PMID: 38617257 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
- R01 AI148477/AI/NIAID NIH HHS/United States
- R21 AI149118/AI/NIAID NIH HHS/United States
- AI149118/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- AI148477/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Medical

