Electrochemical cascade access to hetero[8]circulenes as potent organophotocatalysts for diverse C-X bond formations
- PMID: 40593683
- PMCID: PMC12216941
- DOI: 10.1038/s41467-025-60889-w
Electrochemical cascade access to hetero[8]circulenes as potent organophotocatalysts for diverse C-X bond formations
Abstract
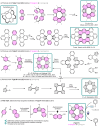
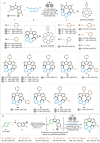
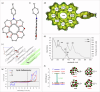
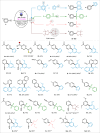
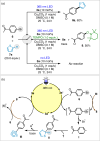
The chemistry of hetero[8]circulenes has been limited to three main types, constrained by synthetic challenges in creating unsymmetrical variants. Herein, we introduce an electrochemical approach to a type of hetero[8]circulene, featuring five hexagons and three pentagons. Our method capitalizes on the sustainability and selectivity of electrochemistry, utilizing differential oxidation potentials to generate dioxaza[8]circulenes through selective intermolecular and intramolecular couplings under mild conditions, achieving yields of up to 83% with good functional group tolerance. We further refine this process into a one-pot protocol using commercially available substrates, forming six new bonds. Comprehensive structural, optical, and electrochemical characterizations, including X-ray crystallography, spectrophotometric analysis, and DFT calculations, are conducted. Inspired by their distinct structural and redox properties, we explore the application of dioxaza[8]circulenes as organophotocatalysts for diverse C-X (X = C, B, S, P) bond formation achieving up to 97% yields under LED light irradiation (365 nm) without transition metals.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
-
- Hensel, T., Andersen, N. N., Plesner, M. & Pittelkow, M. Synthesis of heterocyclic[8]circulenes and related structures. Synlett27, 498–525 (2016).
Grants and funding
LinkOut - more resources
Full Text Sources

