HIV-1 vif mediates ubiquitination of the proximal protomer in the APOBEC3H dimer to induce degradation
- PMID: 40593686
- PMCID: PMC12217271
- DOI: 10.1038/s41467-025-60984-y
HIV-1 vif mediates ubiquitination of the proximal protomer in the APOBEC3H dimer to induce degradation
Abstract
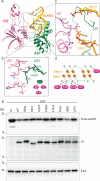
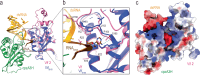
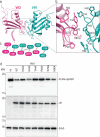
The APOBEC3 family of cytidine deaminases restricts retroviruses like HIV-1 by mutating viral DNA. HIV-1 evades this restriction by producing Vif, which recruits the Cullin-5 (CUL5) E3 ubiquitin ligase complex to promote APOBEC3 degradation. Here we resolve key aspects of this counter-defense mechanism by determining a 3.6 Å cryo-EM structure of chimpanzee APOBEC3H (cpzA3H) in complex with HIV-1 Vif and three components of the CUL5 E3 ligase-CBFβ, EloB, and EloC (VCBC). The structure captures cpzA3H as an RNA-mediated dimer within the cpzA3H-VCBC complex, allowing us to examine the role of dimerization. We find that ubiquitination occurs specifically at two lysine residues on the Vif-proximal protomer, while the distal protomer remains unmodified. The structural model of the active cpzA3H-Vif-CUL5 E3 ligase holoenzyme reveals spatial preferences for ubiquitin transfer to the targeted lysine residues. These findings enhance our understanding of A3H degradation and suggest new antiviral strategies targeting this host-virus interface.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
- R01 AI150478/AI/NIAID NIH HHS/United States
- ZIA BC011627/ImNIH/Intramural NIH HHS/United States
- JP17fk0410304/Japan Agency for Medical Research and Development (AMED)
- JP23fk0410058h0001/Japan Agency for Medical Research and Development (AMED)
- R01AI150478/U.S. Department of Health & Human Services | NIH | Office of Extramural Research, National Institutes of Health (OER)
LinkOut - more resources
Full Text Sources

