Histone serotonylation promotes pancreatic cancer development via lipid metabolism remodeling
- PMID: 40593695
- PMCID: PMC12217961
- DOI: 10.1038/s41467-025-61197-z
Histone serotonylation promotes pancreatic cancer development via lipid metabolism remodeling
Abstract
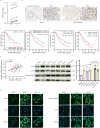
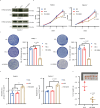
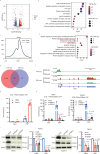
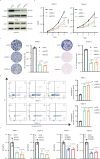
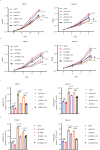
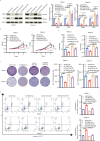
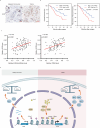
Neurotransmitter serotonin (5-hydroxytryptamine [5-HT]) has emerged to play parallel roles in both neurobiology and oncology. Apart from receptor-mediated signaling transduction pattern, serotonin can be covalently integrated into histone (the post-translational modification known as histone serotonylation) and serve as an epigenetic mark associated with permissive gene expression. However, how histone serotonylation influences tumorigenesis is yet to be understood. In this study, we observe the higher levels of histone serotonylation (H3K4me3Q5ser) and transglutaminases 2 (TGM2, the enzyme catalyzing serotonylation) in both pancreatic ductal adenocarcinoma (PDAC) tissues and cell lines in comparison with their normal counterparts, and inhibition of histone serotonylation suppresses PDAC development. Mechanistically, we demonstrate that TGM2-mediated histone serotonylation at promoter of the gene encoding stearoyl-CoA desaturase (SCD) up-regulates its expression and drives PDAC development by lipid metabolism remodeling. Collectively, this study reveals histone serotonylation as an important driver of PDAC tumorigenesis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
TGM2-mediated histone serotonylation promotes HCC progression via MYC signalling pathway.J Hepatol. 2025 Jul;83(1):105-118. doi: 10.1016/j.jhep.2024.12.038. Epub 2025 Jan 7. J Hepatol. 2025. PMID: 39788430
-
Insights into the mechanisms driven by H3K4 KMTs in pancreatic cancer.Biochem J. 2024 Aug 7;481(15):983-997. doi: 10.1042/BCJ20230374. Biochem J. 2024. PMID: 39078225 Free PMC article. Review.
-
Homeobox protein B6 and homeobox protein B8 control immune-cancer cell interactions in pancreatic cancer.Mol Biomed. 2025 Jul 7;6(1):48. doi: 10.1186/s43556-025-00292-5. Mol Biomed. 2025. PMID: 40619489 Free PMC article.
-
Classical swine fever virus utilizes stearoyl-CoA desaturase 1-mediated lipid metabolism to facilitate viral replication.J Virol. 2025 Jun 17;99(6):e0055125. doi: 10.1128/jvi.00551-25. Epub 2025 May 19. J Virol. 2025. PMID: 40387378 Free PMC article.
-
Roles of lncRNAs in pancreatic ductal adenocarcinoma: Diagnosis, treatment, and the development of drug resistance.Hepatobiliary Pancreat Dis Int. 2023 Apr;22(2):128-139. doi: 10.1016/j.hbpd.2022.12.002. Epub 2022 Dec 14. Hepatobiliary Pancreat Dis Int. 2023. PMID: 36543619
References
-
- Jiang, S. H. et al. Increased Serotonin Signaling Contributes to the Warburg Effect in Pancreatic Tumor Cells Under Metabolic Stress and Promotes Growth of Pancreatic Tumors in Mice. Gastroenterology153, 277–291.e219 (2017). - PubMed
-
- Zhu, P. et al. 5-hydroxytryptamine produced by enteric serotonergic neurons initiates colorectal cancer stem cell self-renewal and tumorigenesis. Neuron110, 2268–2282.e2264 (2022). - PubMed
-
- Jiang, S. H., Hu, L. P., Wang, X., Li, J. & Zhang, Z. G. Neurotransmitters: emerging targets in cancer. Oncogene39, 503–515 (2020). - PubMed
-
- Liu, Y., Zhang, H., Wang, Z., Wu, P. & Gong, W. 5-Hydroxytryptamine1a receptors on tumour cells induce immune evasion in lung adenocarcinoma patients with depression via autophagy/pSTAT3. Eur. J. Cancer114, 8–24 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

