Glial reactivity correlates with synaptic dysfunction across aging and Alzheimer's disease
- PMID: 40593718
- PMCID: PMC12215965
- DOI: 10.1038/s41467-025-60806-1
Glial reactivity correlates with synaptic dysfunction across aging and Alzheimer's disease
Abstract
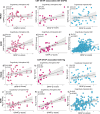
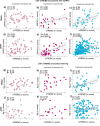
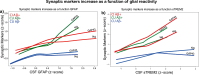
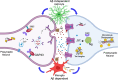
Previous studies suggest glial and neuronal changes may trigger synaptic dysfunction in Alzheimer's disease (AD), but the link between their markers and synaptic abnormalities in the living brain remains unclear. We investigated the association between glial reactivity and synaptic dysfunction biomarkers in cerebrospinal fluid (CSF) from 478 individuals in cognitively unimpaired (CU) and cognitively impaired (CI) individuals. We measured amyloid-β (Aβ), phosphorylated tau (pTau181), astrocyte reactivity (GFAP), microglial activation (sTREM2), and synaptic markers (GAP43, neurogranin). CSF GFAP levels were associated with presynaptic and postsynaptic dysfunction, independent of cognitive status or Aβ presence. CSF sTREM2 levels were related to presynaptic markers in cognitively unimpaired and impaired Aβ+ individuals, and to postsynaptic markers in cognitively impaired Aβ+ individuals. Notably, CSF pTau mediated the relationships between GFAP or sTREM2 and synaptic dysfunction. Our findings, validated in two independent cohorts (TRIAD and ADNI), reveal a distinct pattern of glial contribution to synaptic degeneration.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Therriault has served as a paid consultant for the Neurotorium educational platform and Alzheon. Ashton received payment for lectures from Biogen, BioArctic, Eli-Lilly, and Quanterix. Karikari reported receiving grants from the National Institutes of Health (NIH) and personal fees from the University of Wisconsin–Madison and the University of Pennsylvania outside the submitted work. Additionally, he holds a patent for WO2020193500A1. Zetterberg received personal fees from multiple companies, including AbbVie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave. He also received personal fees for sponsored lectures from Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche outside the submitted work. Furthermore, Zetterberg is a cofounder of Brain Biomarker Solutions, part of the GU Ventures Incubator Program, outside the submitted work. Blennow has served as a consultant and on advisory boards for Acumen, ALZPath, BioArctic, Biogen, Eisai, Eli Lilly and Co, Moleac Pte Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers. He has also served on data monitoring committees for Julius Clinical and Novartis, given lectures, produced educational materials, and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai, and Roche Diagnostics. Additionally, he is a cofounder of Brain Biomarker Solutions, part of the GU Ventures Incubator Program, outside the submitted work. Zimmer served on the Scientific Advisory Board (SAB) of Novo Nordisk, serves on the SAB of Next Innovative Therapeutics (Nintx), and is a cofounder of MASIMA. Rosa-Neto served on the SAB of Novo Nordisk, Eisai, and Eli Lilly, and acted as a consultant for Eisai and Cerveau Radiopharmaceuticals. The remaining authors declare no competing interests.
Figures

Update of
-
Glial reactivity is linked to synaptic dysfunction across the aging and Alzheimer's disease spectrum.Res Sq [Preprint]. 2024 Aug 12:rs.3.rs-4782732. doi: 10.21203/rs.3.rs-4782732/v1. Res Sq. 2024. Update in: Nat Commun. 2025 Jul 1;16(1):5653. doi: 10.1038/s41467-025-60806-1. PMID: 39184102 Free PMC article. Updated. Preprint.
References
-
- Therriault, J. et al. Staging of Alzheimer’s disease: past, present, and future perspectives. Trends Mol. Med.28, 726–741 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

