The human ciliopathy protein RSG1 links the CPLANE complex to transition zone architecture
- PMID: 40593758
- PMCID: PMC12219219
- DOI: 10.1038/s41467-025-61005-8
The human ciliopathy protein RSG1 links the CPLANE complex to transition zone architecture
Erratum in
-
Author Correction: The human ciliopathy protein RSG1 links the CPLANE complex to transition zone architecture.Nat Commun. 2026 Feb 2;17(1):1210. doi: 10.1038/s41467-026-69058-z. Nat Commun. 2026. PMID: 41629334 Free PMC article. No abstract available.
Abstract
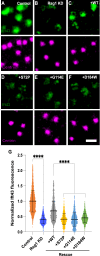
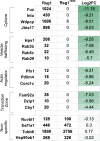
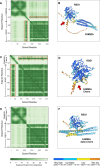
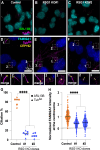
Cilia are essential organelles, and variants in genes governing ciliary function result in ciliopathic diseases. The Ciliogenesis and PLANar polarity Effectors (CPLANE) protein complex is essential for ciliogenesis, and all but one subunit of the CPLANE complex have been implicated in human ciliopathy. Here, we identify three families in which variants in the remaining CPLANE subunit CPLANE2/RSG1 also cause ciliopathy. These patients display cleft palate, tongue lobulations and polydactyly, phenotypes characteristic of Oral-Facial-Digital Syndrome. We further show that these alleles disrupt two vital steps of ciliogenesis, basal body docking and recruitment of intraflagellar transport proteins. Moreover, APMS reveals that Rsg1 binds CPLANE and the transition zone protein Fam92 in a GTP-dependent manner. Finally, we show that CPLANE is generally required for normal transition zone architecture. Our work demonstrates that CPLANE2/RSG1 is a causative gene for human ciliopathy and also sheds new light on the mechanisms of ciliary transition zone assembly.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





Update of
-
The human ciliopathy protein RSG1 links the CPLANE complex to transition zone architecture.bioRxiv [Preprint]. 2024 Sep 26:2024.09.25.614984. doi: 10.1101/2024.09.25.614984. bioRxiv. 2024. Update in: Nat Commun. 2025 Jul 1;16(1):5701. doi: 10.1038/s41467-025-61005-8. PMID: 39386566 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
- R00 HD092613/HD/NICHD NIH HHS/United States
- R01 DK127665/DK/NIDDK NIH HHS/United States
- R03 TR004209/TR/NCATS NIH HHS/United States
- R35 GM122480/GM/NIGMS NIH HHS/United States
- P01 CA254867/CA/NCI NIH HHS/United States
- R01 HD085901/HD/NICHD NIH HHS/United States
- R01 HD089918/HD/NICHD NIH HHS/United States
- R01 GM121565/GM/NIGMS NIH HHS/United States
- R01HD085901/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- K99 HD092613/HD/NICHD NIH HHS/United States
- R01 AR054396/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

